- Submit a Protocol
- Receive Our Alerts
- Log in
- /
- Sign up
- My Bio Page
- Edit My Profile
- Change Password
- Log Out
- EN
- EN - English
- CN - 中文
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
- EN - English
- CN - 中文
- Home
- Protocols
- Articles and Issues
- For Authors
- About
- Become a Reviewer
A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells
Published: Vol 15, Iss 23, Dec 5, 2025 DOI: 10.21769/BioProtoc.5526 Views: 1274
Reviewed by: Olga KopachAnonymous reviewer(s)

Protocol Collections
Comprehensive collections of detailed, peer-reviewed protocols focusing on specific topics
Related protocols

A Hybrid 2D/3D Approach for Neural Differentiation Into Telencephalic Organoids and Efficient Modulation of FGF8 Signaling
Michele Bertacchi [...] Michèle Studer
Jun 20, 2025 2846 Views

Protocol for 3D Bioprinting a Co-culture Skin Model Using a Natural Fibrin-Based Bioink as an Infection Model
Giselle Y. Díaz [...] Stephanie M. Willerth
Jul 20, 2025 3820 Views

Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
Adriana Bajetto [...] Tullio Florio
Feb 5, 2026 205 Views
Abstract
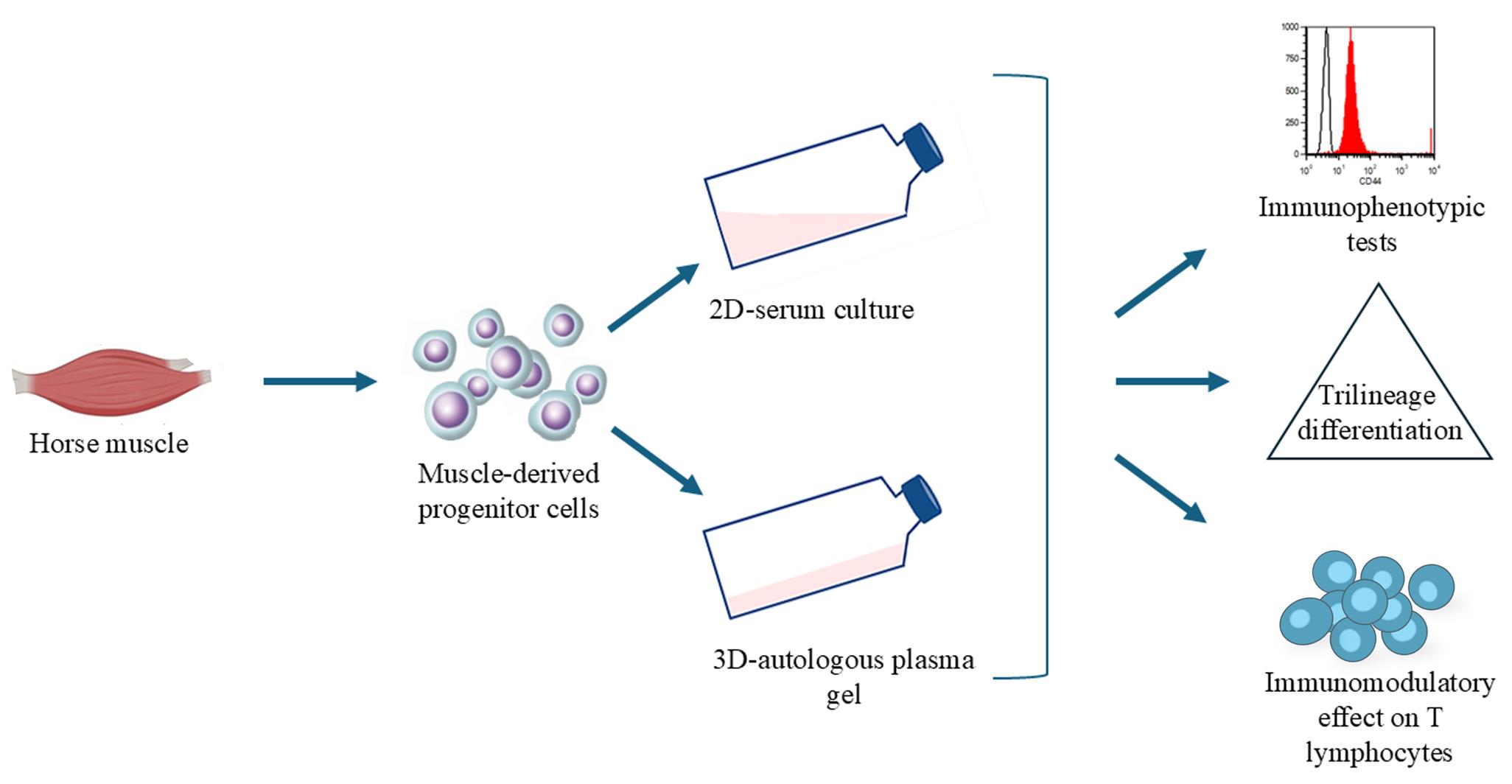
Musculoskeletal pathologies present challenges in athletic horses, often leading to functional impairment. The slow or limited regenerative capacity of bone, joint, and tendon/ligament injuries, coupled with the limitations of conventional treatments, highlights the need for innovative therapies such as ortho-biologics and mesenchymal stem/stroma cells. Traditional 2D cell culture systems with fetal bovine serum (FBS) fail to replicate the complexity of the in vivo environment, whereas 3D cultures more accurately mimic native tissue architecture and cell–cell interactions. This study describes a novel method for isolating muscle-derived progenitor cells in a 3D environment using an autologous plasma-based gel and an innovative cell retrieval solution. The cultured cells exhibit immunomodulatory effects on T lymphocytes, trilineage differentiation potential, and immunophenotypic characteristics consistent with conventional mesenchymal stem/stromal cells. This streamlined 3D culture technique offers a promising platform for generating minimally manipulated autologous cell products tailored for equine regenerative medicine.
Key features
• Development of a simplified autologous 3D-plasma-based culture method, eliminating the need for fetal bovine serum.
• Fully autologous approach offering safer clinical potential for equine regenerative medicine.
• Easy and time-efficient method suitable for researchers familiar with basic cell culture techniques.
Keywords: 3D cell cultureGraphical overview
Background
Musculoskeletal disorders are the leading cause of loss of use in athletic horses. In a study of 126 elite show jumpers, 55% of training days lost for medical reasons were due to non-acute orthopedic injuries and 22% to acute injuries [1]. Prevalence increases with age, affecting 51% of horses over 15 years and up to 77% of geriatric horses over 30 [2]. In aged horses, osteoarthritis and chronic laminitis are most common [3–4]. In Thoroughbreds, fracture risk is markedly elevated, prompting research on early warning markers [5]. Standardbreds show lower fracture incidence but higher rates of tendon and suspensory ligament injuries [6].
The limited healing capacity of bone, joint, tendon, and ligament injuries, coupled with high recurrence rates and modest efficacy of conventional therapies, highlights the need for regenerative strategies. Regenerative medicine aims to restore or replace damaged tissues through approaches such as tissue engineering, stimulation of endogenous repair, or mesenchymal stem cell (MSC) therapy [7–8]. In equine practice, several regenerative modalities—platelet-rich plasma (PRP), autologous conditioned serum (ACS), autologous protein solution (APS), and MSCs—are already in clinical use [9–12]. Adult MSCs have been isolated from multiple tissues, with bone marrow and adipose tissue as the main sources. Muscle-derived MSCs (mdMSCs) have also been described across species [13–16]. Ceusters et al. (2017) introduced a simple sampling method using skeletal muscle microbiopsies. Traditionally, mdMSCs are expanded from explants via density-gradient selection and 2D culture in fetal bovine serum (FBS)-supplemented medium, with FBS being an accepted source of growth factors [17].
However, 2D cultures fail to replicate the architecture and physiological conditions of native tissues [18]. In contrast, 3D cultures provide a more biomimetic environment that better reflects tissue architecture, cell–cell interactions, and functional behavior. Advantages include improved modeling of proliferation, differentiation, disease mechanisms, and drug responses, as well as the ability to study complex processes such as angiogenesis and immune interactions. Nevertheless, 3D systems face challenges: technical complexity, variability across protocols, higher costs, and difficulties in imaging and analysis [19–20]. Recently, our group demonstrated the feasibility of expanding mdMSCs in 2D and 3D cultures using 10% equine allogenic platelet lysate as an alternative to FBS [21].
The present study had two main objectives: (i) to establish a novel method for isolating muscle-derived progenitor cells (mdPg cells) in a 3D plasma-based gel (3D plasma) combined with a safe retrieval solution, thereby addressing the need for simplified culture methods to generate minimally manipulated autologous cell products for equine regenerative medicine; and (ii) to evaluate whether cells cultured under these minimally manipulated 3D-plasma conditions retain key mdMSC properties—namely immunophenotype, immunomodulatory activity, and trilineage differentiation capacity—comparable to conventional 2D serum culture.
Materials and reagents
Reagents
1. Phosphate-buffered saline (PBS) without calcium and magnesium (Gibco, catalog number: 14190)
2. Hank’s balanced salt solution (Gibco, catalog number: 24020)
3. TryPLE Express 1× (Gibco, catalog number: 12604)
4. DMEM/Ham’s F12 medium (Gibco, catalog number: 31330)
5. RPMI-1640 medium (Gibco, catalog number: 21875)
6. Amphotericin B (Gibco, catalog number: 15290)
7. Penicillin-streptomycin (Gibco, catalog number: 15140)
8. Fetal bovine serum (FBS) (Gibco, catalog number: A52568)
9. StemPro® Adipognesis Differentiation kit (Gibco, catalog number: A1007001)
10. StemPro® Chondrogenesis Differentiation kit (Gibco, catalog number: A1007101)
11. StemPro® Osteogenesis Differentiation kit (Gibco, catalog number: A1007201)
12. Phytohemagglutinin (Merck, catalog number: 11249738001)
13. Interleukin 2 Human, 15.5 kDa g/mol (Thermo Scientific, catalog number: J67298.EXE)
14. Paraformaldehyde 4% (Merck, catalog number: 1.00496)
15. Stains: trypan blue, alizarin red, oil red O, and alcian blue (Merck, catalog numbers: T8154, TMS-008, 01391, and B8438, respectively)
16. Diff Quick® stain (Alcyon BeLux, catalog number: 10001615)
17. Cell Collect 3D (Revatis, catalog number: R013D)
18. CD44 (CVS18), CD45 (F10-89-4), and MCHII (CVS20) antibodies (Bio-Rad, catalog numbers: MCA1082GA, MCA87, and MCA1085, respectively)
19. CD90 antibodies (Washington State University, catalog number: DH24A)
20. Goat F(ab')2 anti-mouse IgM mu chain (Abcam, catalog number: Ab5926)
21. FACS running buffer (Miltenyi Biotec, catalog number: 130-092-747)
22. CFDA-SE CellTraceTM CFSE Cell Proliferation kit (ThermoScientific, catalog number: V12883)
23. Mepivacaine 20 mg/mL (Intra-Epicaine, GTIN number: 5701170344646)
Solutions
1. DF-12 (see Recipes)
2. DF-20 (see Recipes)
Recipes
1. DF-12
500 mL of DMEM/Ham’s F12 [containing HEPES (15 mM) and glutamine (2.5 mM)] culture medium, with 5 mL of penicillin (1,000 U/mL)-streptomycin (10,000 μg/mL), and 2.5 mL of amphotericin B (250 µg/mL). Total volume: 507.5 mL.
2. DF-20
500 mL of DMEM/Ham’s F12 culture medium with HEPES and glutamine, 100 mL of FBS, 5 mL of penicillin (1,000 U/mL)-streptomycin (10,000 μg/mL), and 2.5 mL of amphotericin B (250 µg/mL). Total volume: 607.5 mL.
It is recommended to use the assembled culture medium within two months.
Laboratory supplies
1. Cell culture equipment, dishes, flasks and blood tubes (Avantor Belgium)
2. Semiautomatic microbiopsy needles 14G (Medicor Belgium, catalog number: MN-701214090)
3. BD Vacutainer 10 mL PET citrate tube (K2EDTA) for haematology (BD Medical, Becton, Dickinson and Company, catalog number: 367525)
Equipment
1. Flow cytometer (Miltenyi Biotec, model: MACSQuant® Analyzer 10)
2. Inverted microscope (Olympus Corporation, model: CKX53)
Software and datasets
1. Cell count and viability assays were performed using the MedCalc Statistical Software (MedCalc Software Ltd., Ostend, Belgium)
Procedure
A. mdPg cell culture in 3D
Note: In our work, five horses were used as donors: two geldings and three mares, with ages ranging from 8 to 22 years. The age and sex of the donor horses were not considered critical variables and were not expected to influence the experimental procedures. Microbiopsy procedures on animals were approved by the Animal Ethical Commission of the University of Liège (N° 2421) and performed following the relevant guidelines. Muscle microbiopsy samples were obtained from the triceps brachii (Figure 1) after subcutaneous application of 1 mL of mepivacaine (20 mg/mL) and then stored at 4 °C in DF-12 medium for a maximum of 72 h before initiating the culture. In addition to microbiopsy, for each horse, whole blood (40 mL) was collected on citrate tubes and then centrifuged at 2,000× g for 15 min. Plasma was collected, aliquoted, and frozen at -20 °C until use.

Figure 1. Injection of mepivacaine into the triceps brachii muscle of a horse, prior to the muscle sampling procedure, which is performed using a semiautomatic microbiopsy 14G needle
1. Prepare the culture using sterile equipment under a streamlined flow hood.
2. Cut the microbiopsy specimens into small pieces (1–2 mm) and place each piece individually into the 16 central wells of a 24-well multiwell dish.
3. Fill each well with 400 μL of DF-12 enriched with 20% autologous blood plasma. Under these conditions, the culture medium solidifies within 30 min, and proliferating cells distribute within the coagulated 3D plasma.
4. Do not attempt to completely replace the medium; instead, add medium every 3–4 days to supply nutrients and prevent the plasma gel from drying out.
5. Incubate the multiwell dish at 37 °C in a controlled atmosphere (5% CO2) until cells migrate out of the explants (typically after 4–7 days).
6. Allow approximately 20 days to obtain optimal cellular confluence around the explant (70%–90% confluence).
7. As a control, initiate cultures in 2D serum conditions using DF-12 supplemented with 20% FBS (DF-20), following the protocol described by Ceusters et al. [17].
B. mdPg cell amplification in 3D
1. After approximately 10 days of explant culture, harvest confluent cells. Do not use conventional enzyme detachment methods (e.g., trypsin), as the cells are cultivated in a 3D environment. Instead, following the manufacturer’s instructions, add a gel dissolution solution (Cell Collect 3D) to the culture and incubate for ~40 min at room temperature to liberate the cells from the coagulated mass.
2. After dissolution, extract the content of each well and transfer it to a conical tube.
3. Centrifuge at 300× g for 10 min at room temperature.
4. Discard the supernatant, leaving the cells at the bottom of the tube.
5. Add 5 mL of DF-12 medium and homogenize the suspension by several aspiration–reinjection cycles.
6. Seed the resulting cell suspension in one 175 cm2 T-flask containing 20 mL of DF-12 supplemented with 20% plasma to allow further multiplication of mdPg cells.
7. Allow coagulation to occur (within ~30 min).
8. Incubate the flask at 37 °C in 5% CO2 until 70%–90% confluence is achieved (~10 days).
9. Do not renew the plasma gel. Instead, add medium every 3–4 days to prevent nutrient depletion and maintain cell viability. Since the medium cannot be completely replaced, supplement the culture with both plasma and medium to ensure sufficient nutrient supply. For a 175 cm2 T-flask, add 10 mL of DF-12 and 20% plasma. See Figure 2 for the start of gel colonization by the cells.

Figure 2. Microscopic pictures (inverted microscope, 100×) of cells in 3D-plasma gel coming out from muscle explants
C. Growth curves of 3D cultured cells
1. Use 25 cm2 T-flasks with 5 mL of DF-12 to establish a growth curve of the cells in 3D-plasma culture.
2. Seed 200,000 mdPg cells in each flask and add 20% plasma. For control data, seed 200,000 mdMSCs obtained from the same five donors using the technology described by Ceusters et al. [17]. Culture the control cells in 2D serum with DF-12 supplemented with 20% FBS (DF-20).
3. Incubate the flasks at 37 °C in 5% CO2. Do not perform additional maintenance, as the cells remain dispersed in the plasma.
4. For cell counting, dissolve the gel with Cell Collect 3D following the manufacturer’s instructions and harvest the cells in HBSS.
5. Count the cells manually using a Bürker chamber and assess viability with trypan blue dye.
6. Repeat this procedure every 2 days from culture initiation for a total of 10 days to evaluate cell growth and viability.
7. Compare the two growth curves (n = 5) using a two-factorial study (time and medium) with repeated measures (MedCalc, Belgium).
D. Cell morphology
1. Evaluate cell morphologies in the 3D-plasma gel with Diff Quick® staining at different steps of the process.
2. Compare cell morphologies with those of cells cultured in 2D serum with DF-20 (Figure 3).

Figure 3. Microscopic photographs (optical microscope, 100×) of cells cultured in (A) 3D plasma and (B) 2D serum culture media after Diff Quick® staining
E. Immunophenotyping
1. Analyze the cells cultured in 3D-plasma gel by flow cytometry using a MACSQuant 10.
a. Harvest the cells as previously described, wash with PBS, and centrifuge at 600× g for 5 min.
b. Resuspend the cell pellets in 500 µL of FACS running buffer. Owing to the high specificity of the antibodies used, it is unnecessary to perform a fixation and blocking step prior to staining.
c. Incubate the cells with conjugated antibodies (see Table 1) for 15 min at 4 °C in the dark: CD44/FITC, CD45/PerCP, and MHCII/PE. For CD90, incubate with the unconjugated primary anti-CD90 for 15 min at 4 °C in the dark, followed by a secondary FITC-coupled antibody (Anti-IgM) under the same conditions.
d. After incubation, dilute the cells with FACS running buffer and centrifuge at 600× g for 5 min.
e. Wash the samples twice with FACS running buffer.
f. Perform data acquisition on the MACSQuant 10 Analyzer.
2. For comparison, collect cells from 3D plasma and start a 2D-serum condition culture (DF-20) for 24 h. Detach the cells using TrypLE Express and subject them to the same flow cytometry analysis.
Table 1. Antibodies used for flow cytometry analysis
| Antibodies | Supplier | Catalog number | Dilution |
|---|---|---|---|
| CD90:DH24A IgM | Washington State University-Monoclonal Antibody Center | DH24A | 50 |
| Mouse anti-human CD45:PerCP | Bio-Rad | MCA87PERCP | 5 |
| MHC Class II Monomorphic Antibody: PE | Bio-Rad | MCA1085PE | 25 |
| Goat F(ab')2 anti-mouse IgM mu chain (FITC) | Abcam | Ab5926 | 25 |
| Mouse anti-horse CD44 : FITC | Bio-Rad | MCA1082F | 25 |
Note: Cells cultured in 3D plasma were positive for CD90 (>90%) and negative for MHCII and CD45 (both <2%) (data not shown). The CD44 marker is also negative for the cells cultured in 3D (<7%). Interestingly, when transferred to 2D-serum culture for 24 h, these cells re-expressed CD44 (>90%) while maintaining CD90 positivity and negativity for CD45 and MHCII (Figure 4).

Figure 4. Change in CD44 expression of cells cultured in (A) 3D-plasma medium first and (B) replaced with 2D-serum culture medium for 24 h. Unfilled histograms correspond to the control population (unmarked).
F. Trilineage differentiations
1. Seed the cells generated in 3D-plasma culture at a density of 200,000 cells/well in a 24-well plate using DF-20 culture medium under classical 2D culture conditions.
2. Upon reaching confluence, replace the complete medium with StemPro® chondrogenesis, osteogenesis, or adipogenesis differentiation medium for the induction of chondroblasts, osteoblasts, and adipocytes, respectively.
3. For negative controls, maintain cells in 3D culture (DF-12 + 20% blood plasma) and in classical 2D-serum culture (DF-20).
4. Incubate the plate for 21 days at 37 °C in a 5% CO2 incubator. Replace the differentiation medium once per week, following the manufacturer’s instructions.
5. After incubation, fix the differentiated cells with 4% paraformaldehyde for 15 min.
6. Stain with alcian blue, alizarin red, or oil red O for 15 min at room temperature to evaluate the presence of chondroblasts (mucopolysaccharides in cartilage matrix), osteoblasts (calcium deposits), and adipocytes (lipid content and triglycerides), respectively (Figure 5).
G. Immunomodulatory capacities of 3D-plasma cultured cells
Note: This section aims to answer the question: Do cells cultured in 3D systems retain immunomodulatory capabilities comparable to those cultured in 2D systems?
1. Collect mdPg cells cultured in 3D plasma from three different horses and plate them at 4,000 cells/cm2 in a flat-bottom 24-well plate with RPMI-1640 medium supplemented with 10% FBS.
2. Allow a period for cell adherence (>4 h).
3. Add allogeneic T lymphocytes (T cells) purified from peripheral horse blood and stimulated with 5 µg/mL phytohemagglutinin (PHA) and 50 U/mL IL-2.
4. Incubate the co-cultures for 5 days in RPMI-1640 medium supplemented with 10% FBS.
5. Test different mdPg cell:T cell ratios (ranging from 1:80 to 1:1) to evaluate their impact on stem cell–mediated effects.
6. Evaluate T-lymphocyte proliferation by CFDA-SE labeling. For labeling, stain 107 T cells with 10 mM CFDA-SE (from CellTraceTM CFSE Cell Proliferation kit) for 10 min at 37 °C in the dark, prior to co-incubation with mdPg cells.
7. After 5 days of co-culture, analyze CFSE fluorescence by flow cytometry. Identify T-cell proliferation by the appearance of multiple peaks corresponding to successive cell divisions. Express T-cell proliferation as the percentage of dividing lymphocytes compared to the control condition without mdPg cell contact.

Figure 5. Microscopic photographs (optical microscope, 100×) of cells cultured in 3D plasma (G, H, I) and 2D serum medium (D, E, F) differentiated in the three lineages. The left column (D, G) shows differentiation into chondroblasts, evidenced by alcian blue staining. Cells undergoing chondroblastic differentiation formed spheroids. The central column (E, H) depicts differentiation into osteoblasts, with calcium deposits stained deep red by alizarin red. The right column (F, I) represents adipocyte differentiation, highlighted by small lipid deposits stained red-orange with oil red O. The top row (A, B, C) corresponds to undifferentiated cells cultured in 3D. All images were captured at the same magnification.
Data analysis
Cells were cultured using two methods: 2D culture supplemented with fetal FBS and 3D culture supplemented with plasma. Cell counts were performed every 48 h to generate growth curves. At equal seeding density (200,000 cells), cells in 3D-plasma medium showed a trend toward higher proliferation than those in 2D-serum medium (P = 0.16). After 10 days, cell counts reached ~1.4 million in 3D plasma vs. ~1.2 million in 2D serum (Figure 6). Data were analyzed using ANOVA, with a sample size of n = 5. Plasma or serum was the only variable between conditions. Viability was comparable: 89.2% (±2.05) in 2D serum and 88.4% (±2.88) in 3D plasma.

Figure 6. Growth curves of cells cultured in 3D plasma medium compared to 2D cultures maintained in serum medium. Cells were cultured for a total of 10 days. At each time point, selected flasks were harvested, and cells were detached and counted to determine growth progression. For each culture condition (n = 5), cell counts were performed in triplicate (mean +/- SD).
Cells cultured in 3D systems from the plasma of three horses were co-cultured with allogeneic T lymphocytes activated by PHA and IL-2, at varying ratios. T-cell proliferation was measured by CFDA-SE labeling and flow cytometry after 5 days. The results showed that 3D plasma-derived cells inhibited T-lymphocyte proliferation in a dose-dependent manner relative to the mdPg cell:T ratio (Figure 7). Cells cultured in 3D retain immunomodulatory capabilities that can suppress T-cell proliferation effectively.

Figure 7. Representative curve showing the inhibition of activated T-cell proliferation by cells cultured in 3D plasma (n = 3)
This study introduces a simplified 3D-plasma culture system for generating minimally manipulated autologous progenitor cells. Compared to conventional 2D-FBS culture, the method is faster, technically simpler, and avoids animal-derived supplements. Autologous plasma serves both as scaffold and growth factor source, reducing risks associated with FBS or allogeneic products, such as immune reactions or regulatory constraints.
Cells expanded in 3D plasma retained MSC-like characteristics: immunophenotype, trilineage differentiation, and immunomodulatory properties, including ratio-dependent inhibition of T-cell proliferation (>60% at 1:1). Importantly, cells grew without passaging, potentially limiting senescence, and their retrieval was achieved using an enzymatic gel-dissolution step without altering surface markers.
In summary, the 3D-plasma system provides a simple, physiologically relevant, and fully autologous culture platform for muscle-derived progenitor cells. The technique yields cells with MSC features and therapeutic potential, supporting its further development toward standardized regenerative applications.
Validation of protocol
This protocol, or portions thereof, has been employed and validated in the following research article involving a subpopulation of equine muscle-derived cells (flow cytometry analyses, trilineage differentiation assays, and proliferation inhibition studies).
Ceusters et al. [17]. From skeletal muscle to stem cells: an innovative and minimally-invasive process for multiple species. Scientific Reports.
General notes and troubleshooting
General notes
1. When collecting blood samples, gently invert freshly filled tubes several times to prevent coagulation.
2. For plasma and FBS, avoid repeated freeze-thaw cycles as much as possible. It is recommended to prepare aliquots to minimize degradation.
3. One hour after seeding cells in 3D culture, assess the gelation of the culture medium. Incomplete or failed solidification may result from plasma that has been frozen and thawed too many times (>6 times) or from calcium deficiency. The typical calcium concentration in the culture medium is approximately 1.8 mM.
Acknowledgments
All co-authors participated in the writing and reviewing of the manuscript.
H.G. and J.D.: Principal investigators responsible for the project and overseeing the follow-up of each assay.
J.C.: Supervised the FACS analyses and contributed to protocol development.
D.S. and C.S.: Performed muscle microbiopsies and provided suggestions for result analysis.
A.N., T.F., and A.M.: Conducted laboratory manipulations and adapted protocols based on the results.
Competing interests
D. Serteyn and J. Ceusters are the co-inventors of a patent related to muscle-derived stem cells. This patent is licensed by the University of Liège to Revatis, a Spin-Off company where D. Serteyn and J. Ceusters serve as scientific advisors. The other co-authors declare no conflicts of interest.
Ethical considerations
Muscle microbiopsy protocol for producing MSC/mdPg cells was approved by the ethics committee of the University of Liege (N° 21–2421).
References
- Jönsson, L., Näsholm, A., Roepstorff, L., Egenvall, A., Dalin, G. and Philipsson, J. (2013). Genetic analysis of clinical findings at health examinations of young Swedish warmblood riding horses. Acta Vet Scand. 55(1): e1186/1751–0147–55–22. https://doi.org/10.1186/1751-0147-55-22
- Ribitsch, I., Oreff, G. L. and Jenner, F. (2021). Regenerative Medicine for Equine Musculoskeletal Diseases. Animals. 11(1): 234. https://doi.org/10.3390/ani11010234
- Ireland, J. L., McGowan, C. M., Clegg, P. D., Chandler, K. J. and Pinchbeck, G. L. (2012). A survey of health care and disease in geriatric horses aged 30years or older. Vet J. 192(1): 57–64. https://doi.org/10.1016/j.tvjl.2011.03.021
- van Weeren, P. R. and Back, W. (2016). Musculoskeletal Disease in Aged Horses and Its Management. Vet Clin N Am: Equine Pract. 32(2): 229–247. https://doi.org/10.1016/j.cveq.2016.04.003
- Wright, I., Minshall, G., Young, N. and Riggs, C. (2024). Fractures in Thoroughbred racing and the potential for pre‐race identification of horses at risk. Equine Vet J. 56(3): 424–436. https://doi.org/10.1111/evj.14046
- Bertuglia, A., Bullone, M., Rossotto, F. and Gasparini, M. (2014). Epidemiology of musculoskeletal injuries in a population of harness Standardbred racehorses in training. BMC Vet Res. 10(1): 11. https://doi.org/10.1186/1746-6148-10-11
- Sousa, B. R., Parreira, R. C., Fonseca, E. A., Amaya, M. J., Tonelli, F. M. P., Lacerda, S. M. S. N., Lalwani, P., Santos, A. K., Gomes, K. N., Ulrich, H., et al. (2013). Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytometry Part A. 85(1): 43–77. https://doi.org/10.1002/cyto.a.22402
- Shammaa, R., El-Kadiry, A. H., Abusarah, J. and Rafei, M. (2020). Mesenchymal Stem Cells Beyond Regenerative Medicine. Front Cell Dev Biol. 8: e00072. https://doi.org/10.3389/fcell.2020.00072
- Geburek, F., Gaus, M., van Schie, H. T. M., Rohn, K. and Stadler, P. M. (2016). Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies – a randomized prospective controlled clinical trial. BMC Vet Res. 12(1): e1186/s12917–016–0826–1. https://doi.org/10.1186/s12917-016-0826-1
- Frisbie, D. D., Kawcak, C. E., Werpy, N. M., Park, R. D. and McIlwraith, C. W. (2007). Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 68(3): 290–296. https://doi.org/10.2460/ajvr.68.3.290
- Bertone, A. L., Ishihara, A., Zekas, L. J., Wellman, M. L., Lewis, K. B., Schwarze, R. A., Barnaba, A. R., Schmall, M. L., Kanter, P. M., Genovese, R. L., et al. (2014). Evaluation of a single intra-articular injection of autologous protein solution for treatment of osteoarthritis in horses. Am J Vet Res. 75(2): 141–151. https://doi.org/10.2460/ajvr.75.2.141
- Renzi, S., Riccò, S., Dotti, S., Sesso, L., Grolli, S., Cornali, M., Carlin, S., Patruno, M., Cinotti, S., Ferrari, M., et al. (2013). Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res Vet Sci. 95(1): 272–277. https://doi.org/10.1016/j.rvsc.2013.01.017
- Stewart, A. A., Barrett, J. G., Byron, C. R., Yates, A. C., Durgam, S. S., Evans, R. B. and Stewart, M. C. (2009). Comparison of equine tendon-, muscle-, and bone marrow–derived cells cultured on tendon matrix. Am J Vet Res. 70(6): 750–757. https://doi.org/10.2460/ajvr.70.6.750
- Lecourt, S., Marolleau, J. P., Fromigué, O., Vauchez, K., Andriamanalijaona, R., Ternaux, B., Lacassagne, M. N., Robert, I., Boumédiene, K., Chéreau, F., et al. (2010). Characterization of distinct mesenchymal-like cell populations from human skeletal muscle in situ and in vitro. Exp Cell Res. 316(15): 2513–2526. https://doi.org/10.1016/j.yexcr.2010.04.020
- Kisiel, A. H., McDuffee, L. A., Masaoud, E., Bailey, T. R., Esparza Gonzalez, B. P. and Nino-Fong, R. (2012). Isolation, characterization, and in vitro proliferation of canine mesenchymal stem cells derived from bone marrow, adipose tissue, muscle, and periosteum. Am J Vet Res. 73(8): 1305–1317. https://doi.org/10.2460/ajvr.73.8.1305
- Radtke, C. L., Nino-Fong, R., Esparza Gonzalez, B. P., Stryhn, H. and McDuffee, L. A. (2013). Characterization and osteogenic potential of equine muscle tissue– and periosteal tissue–derived mesenchymal stem cells in comparison with bone marrow– and adipose tissue–derived mesenchymal stem cells. Am J Vet Res. 74(5): 790–800. https://doi.org/10.2460/ajvr.74.5.790
- Ceusters, J., Lejeune, J. P., Sandersen, C., Niesten, A., Lagneaux, L. and Serteyn, D. (2017). From skeletal muscle to stem cells: an innovative and minimally-invasive process for multiple species. Sci Rep. 7(1): e1038/s41598–017–00803–7. https://doi.org/10.1038/s41598-017-00803-7
- Fontoura, J. C., Viezzer, C., dos Santos, F. G., Ligabue, R. A., Weinlich, R., Puga, R. D., Antonow, D., Severino, P. and Bonorino, C. (2020). Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance. Materials Science and Engineering: C. 107: 110264. https://doi.org/10.1016/j.msec.2019.110264
- Antoni, D., Burckel, H., Josset, E. and Noel, G. (2015). Three-Dimensional Cell Culture: A Breakthrough in Vivo. Int J Mol Sci. 16(3): 5517–5527. https://doi.org/10.3390/ijms16035517
- Centeno, E. G. Z., Cimarosti, H. and Bithell, A. (2018). 2D versus 3D human induced pluripotent stem cell-derived cultures for neurodegenerative disease modelling. Mol Neurodegener. 13(1): e1186/s13024–018–0258–4. https://doi.org/10.1186/s13024-018-0258-4
- Duysens, J., Graide, H., Niesten, A., Mouithys-Mickalad, A., Deby-Dupont, G., Franck, T., Ceusters, J. and Serteyn, D. (2024). Culture and Immunomodulation of Equine Muscle-Derived Mesenchymal Stromal Cells: A Comparative Study of Innovative 2D versus 3D Models Using Equine Platelet Lysate. Cells. 13(15): 1290. https://doi.org/10.3390/cells13151290
Article Information
Publication history
Received: Aug 28, 2025
Accepted: Oct 22, 2025
Available online: Nov 11, 2025
Published: Dec 5, 2025
Copyright
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
How to cite
Graide, H., Duysens, J., Frank, T., Mouithys-Mickalad, A., Niesten, A., Sandersen, C., Ceusters, J. and Serteyn, D. (2025). A Simplified 3D-Plasma Culture Method for Generating Minimally Manipulated Autologous Equine Muscle-Derived Progenitor Cells. Bio-protocol 15(23): e5526. DOI: 10.21769/BioProtoc.5526.
Category
Stem Cell > Adult stem cell > Muscle stem cell
Cell Biology > Cell isolation and culture > 3D cell culture
Stem Cell > Pluripotent stem cell > Regenerative medicine
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link








