- EN - English
- CN - 中文
Introducing Exogenous DNA Vectors Directly into Trypoxylus dichotomus Larvae Via In Vivo Electroporation
发布: 2026年02月20日第16卷第4期 DOI: 10.21769/BioProtoc.5602 浏览次数: 39
评审: Anonymous reviewer(s)
Abstract
In the Japanese rhinoceros beetle Trypoxylus dichotomus, gene function studies have relied mainly on systemic larval RNA interference (RNAi), as gain-of-function techniques remain underdeveloped and germline transgenesis is impractical given the species’ approximately one-year generation time. In addition, because larval RNAi is systemic, it has been difficult to analyze the function of lethal genes. Here, we present a simple and efficient protocol for the direct introduction of exogenous DNA into T. dichotomus larvae via in vivo electroporation. This protocol includes optimized procedures for adult breeding and egg collection, as well as a rigorously parameterized electroporation technique that delivers a piggyBac transposon vector into region-specific larval tissues. Within one day after electroporation, treated larvae exhibit mosaic expression of a reporter gene, enabling rapid tissue-specific functional analysis without the need to establish stable germline transgenic lines. Moreover, the key promoter used in this system (T. dichotomus actinA3 promoter) is effective across diverse insect species, indicating that the method can be readily adapted to other non-model insects. Overall, this electroporation-based approach provides a valuable gain-of-function tool for T. dichotomus and potentially many other insect species.
Key features
• A simple and efficient method for in vivo electroporation of T. dichotomus larvae.
• Applicable to other insect species.
• The T. dichotomus actinA3 promoter functions effectively across a diverse range of insect species.
• An optimized method for egg collection in T. dichotomus.
Keywords: ElectroporationBackground
Electroporation transiently permeabilizes cell membranes with electrical pulses, enabling intracellular delivery of nucleic acids and other macromolecules [1–3]. In vivo applications have supported tissue-specific gene function analyses in vertebrates and multiple insects [4–11]. Electroporation is compatible with both gain and loss-of-function assays and genetic mosaic analysis within a single organism [9]. The Japanese rhinoceros beetle, Trypoxylus dichotomus (Coleoptera, Scarabaeidae, Dynastini) (Figure 1), is a large insect species reaching up to 90 mm in body length. In T. dichotomus, molecular studies still rely heavily on systemic larval RNAi [12–16]. Gain-of-function and region-specific approaches remain underdeveloped, and germline transgenesis or genome editing is impractical due to the 10–12-month generation time [17]. In addition, because larval RNAi is systemic, it has been difficult to analyze the function of lethal genes. Therefore, electroporation is the optimal technique for gain-of-function and region-specific approaches in T. dichotomus. In large-bodied insects such as T. dichotomus, however, the efficiency of delivery of exogenous DNA vectors via electroporation depends critically on developmental stage, body size, electrode geometry/placement, pulse parameters, and cargo properties, underscoring the need for standardized, reproducible protocols. We present a rigorously parameterized, region-specific gain-of-function in vivo electroporation protocol for T. dichotomus larvae, optimized for reproducibility [18].

Figure 1. Trypoxylus dichotomus. (A) Adult male and (B) female T. dichotomus. Scale bar: 10 mm.
Materials and reagents
1. Filter pipette tips (RNase/DNase-free) [Labcon, catalog number: 1055-965-018-9 (20 μL) and QSP, catalog number: TF102-10-Q (2 μL)]
2. Microloader (Eppendorf, catalog number: 2229001206)
3. 1.5 mL microtubes, flat-bottom, DNase/RNase-free (Watson, catalog number: 131-415C)
4. 0.2 mL Flat PCR tube 8-cap strips (INA OPTIKA, catalog number: 3247-00)
5. Lavender nitrile powder-free exam gloves (Kimberly-Clark, catalog number: 52818)
6. Plasmid pBac[Tdic-actA3-nls-EGFP_Dmel-hsp70-DsRed2] (Figure 2) [18]
7. Plasmid pBac[phsp-pBac] [19]
8. UltraPureTM DNase/RNase-free distilled water (UPW) (Invitrogen, catalog number: 10977023)
9. Blow container 750 cc (Hobby Club, https://www.hobby-club.jp/items/25101620)
10. Humus (Dorcus Owner’s shop, https://www.dorukusu.com/item/847/)
11. Pro-jelly 16 g (KB farm, https://www.wraios.co.jp/zeri.htm)
12. Jelly Splitter II (Coelacase, https://www.din.or.jp/~coelacan/n_sp.html)
13. Clean cup (Risupack, catalog number: 200B)
14. Spoon (stainless steel) 180 mm (AS ONE, catalog number: 6-522-04)
15. Bamboo chopsticks (generic)
16. Insect pins (Shigakontyu, model: INSECT PINS, No. 3)
17. Nylon mesh cloth (70 mesh) (NBC Meshtec, catalog number: T-NO.70S)
18. Container [454 mm (L) × 292 mm (W) × 250 mm (H)] (Fudogiken, catalog number: NUE250)
19. Tray [860 mm (L) × 530 mm (W) × 150 mm (H)] (Sanko, catalog number: 673015)
20. Yogurt crate H-20-3 (Sanko, catalog number: 200758)
21. Ultrasound gel, LOGIQLEAN HARD (GE HealthCare, catalog number: 2369385)
22. Glass capillary with a scale (NARISHIGE, model: GDC-1; scale added in 0.6 mm increments by Daiwa Union Co., Ltd., Japan)
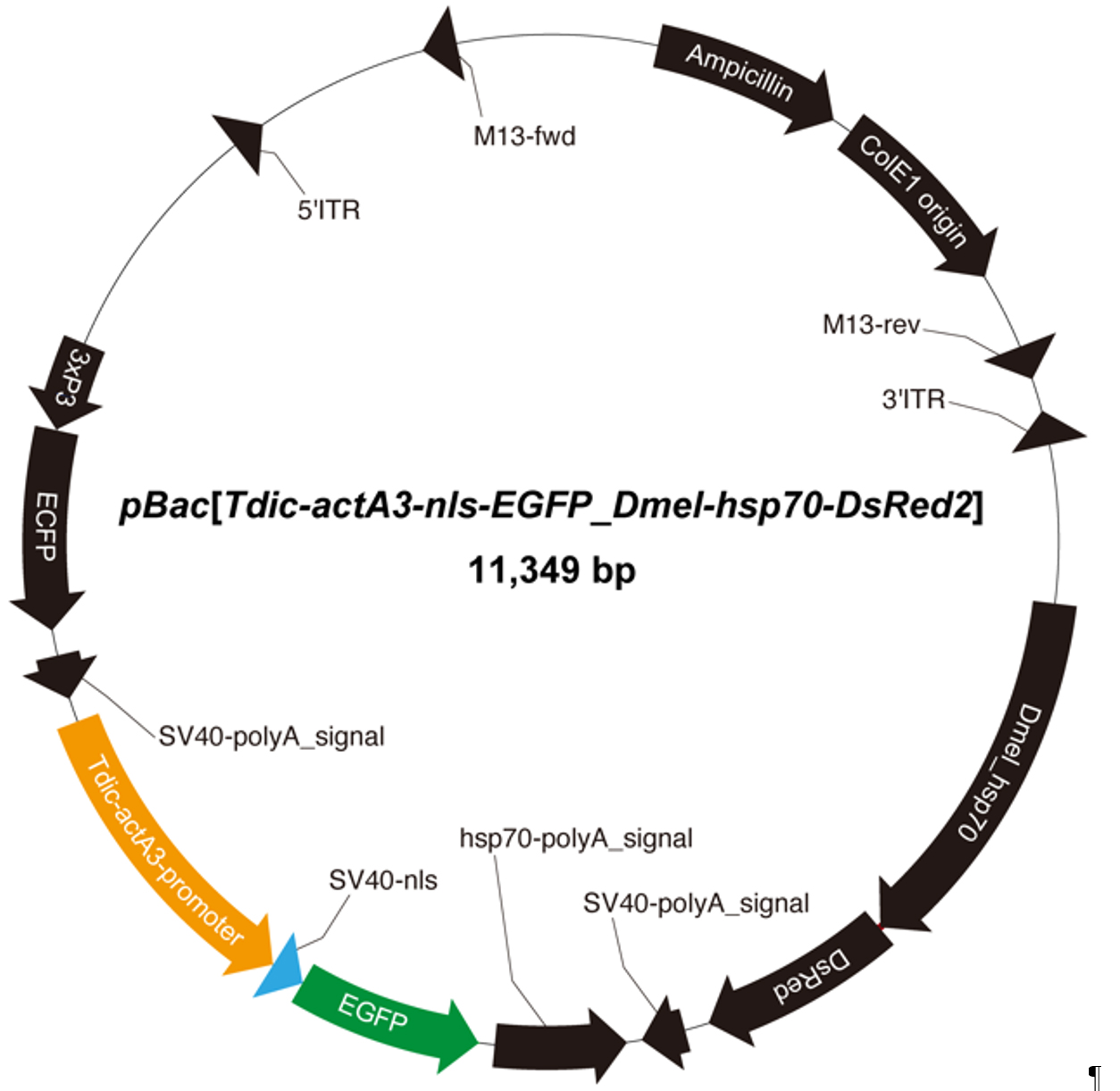
Figure 2. Schematic of the plasmid vector for piggyBac transformation of T. dichotomus. To establish an in vivo electroporation method in T. dichotomus tissues, we employ the promoter of the actin A3 gene, which is ubiquitously expressed [18]. This vector also contains a DsRed2 expression cassette driven by the Drosophila hsp70 promoter, which is active in various insects—including the silkworm Bombyx mori [20], the red flour beetle Tribolium castaneum [21], and the butterflies Bicyclus anynana [22] and Papilio xuthus [9]—as well as an EGFP expression cassette driven by the 3xP3 promoter. NLS indicates a nuclear localization signal.
Equipment
1. Micropipette [Gilson, catalog number: F144054M (P2), F144056M (P20)]
2. Centrifuge (TOMY, model: MX-307)
3. Micropipette puller (Sutter Instrument, model: P-97/IVF)
4. Micropipette grinder (NARISHIGE, model: EG-400)
5. Microinjector (Eppendorf, model: FemtoJet 4i)
6. Fluorescent stereomicroscope (Nikon, model: SMZ18)
7. Electroporation electrodes [NEPAGENE, model: CUY665Ti9-2.5-0.5 (0.5 mm), CUY650P1 (1 mm), CUY650P3 (3 mm), CUY650P10 (10 mm)]
8. Electroporator (NEPAGENE, model: NEPA21 Type II)
9. Manipulator (NARISHIGE, model: M-152, HI-7)
Procedure
文章信息
稿件历史记录
提交日期: Nov 18, 2025
接收日期: Jan 12, 2026
在线发布日期: Jan 22, 2026
出版日期: Feb 20, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Morita, S. and Niimi, T. (2026). Introducing Exogenous DNA Vectors Directly into Trypoxylus dichotomus Larvae Via In Vivo Electroporation. Bio-protocol 16(4): e5602. DOI: 10.21769/BioProtoc.5602.
分类
发育生物学
分子生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









