- EN - English
- CN - 中文
Low Angle Ring Illumination Stereomicroscopy (LARIS) Method for High-Contrast Imaging of Drosophila Compound Eyes
低角度环形照明体视显微成像(LARIS)方法用于果蝇复眼的高对比度成像
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5590 浏览次数: 135
评审: Shweta YadavAnonymous reviewer(s)
Abstract
The compound eyes of Drosophila are widely used to gain valuable insights into genetics, developmental biology, cell biology, disease biology, and gene regulation. Various parameters, such as eye size, pigmentation loss, formation of necrotic patches, and disorientation, fusion, or disruption of ommatidial arrays, are commonly assessed to evaluate eye development and degeneration. We developed an improved imaging method named low-angle ring illumination stereomicroscopy (LARIS) to capture high-contrast images of the Drosophila compound eye. Different optical alignments were tested to capture the fly compound eye image under the stereomicroscope; the highest contrast with minimal reflection was achieved through the LARIS method. The images captured using LARIS clearly showed ommatidial fusion, disorientation, and pigmentation loss, which were hardly visible with a conventional imaging method in the degenerating compound eyes of Drosophila. In addition to its research applications, this protocol is cost-effective due to the low expenses associated with supplies and equipment. We anticipate that LARIS will facilitate high-contrast imaging of the compound eyes in Drosophila and other insects.
Key features
• Low-angle ring illumination stereomicroscopy (LARIS) is an improved optical alignment for high-contrast imaging of Drosophila compound eyes.
• LARIS is a simple, inexpensive, and robust method with additional advantages over existing SEM methods to capture high-contrast microscopic images of the Drosophila eye.
• LARIS can be easily implemented across laboratories and used as a low-cost teaching/research tool.
Keywords: Eye development (眼发育)Graphical overview
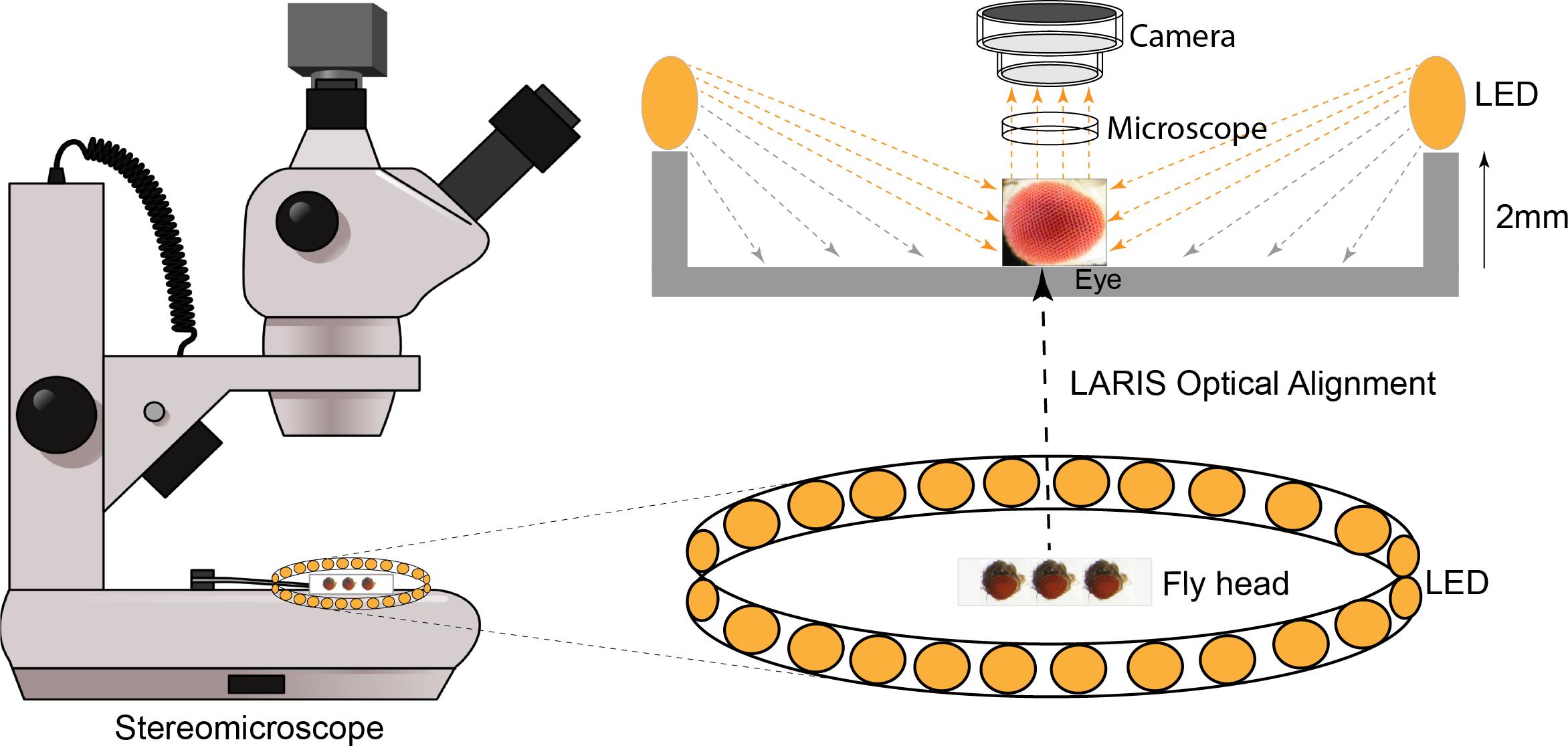
Background
The Drosophila compound eye is comprised of approximately 750 ommatidia that are packed into a remarkably ordered three-dimensional ommatidial array covered with transparent cuticles secreted by cone cells [1]. This layer scatters light, which makes it challenging to capture high-contrast eye images using a stereomicroscope equipped with a standard illumination system. Analysis of such images by a trained researcher or an automated image analysis system can only give an approximation with the possibility of misinterpretation [2,3]. Scanning electron microscopy (SEM) is a popular technique for high-resolution imaging of the eye, but it requires imaging skills and resources [4,5]. Furthermore, SEM images provide no information about pigmentation alteration in the adult fly eye. Nail polish imprint is another high-contrast imaging technique to image the molds of the fly eye, but this technique requires hand skill and does not provide pigmentation details [6,7].
To fix this problem, we tested different light sources, including a bar light, a gooseneck light, a diffused/straight backlight, and a ring light to illuminate the eye under a stereomicroscope. Images of bar- or gooseneck light–illuminated eyes had considerable glare with shaded regions (Figure 1A). The dome light alignment, which provided even and diffused light, generated less glare with slightly improved image contrast (Figure 1B). The transmitted light source provided some structural details, but the contrast was poor (Figure 1C). The ring illuminator, fitted around the objective lens of the stereomicroscope, provided a cylinder of light, illuminating the eye from the top. It generated an image with no unwanted shades but an intense glare on the ommatidia (Figure 1D). Also, the ring illumination images provided better contrast but lacked details of the ommatidial structure. We removed the ring illuminator from the objective lens assembly and positioned it closer to the fly on the stereobinocular stage. The glare disappeared when the light source and fly were at the same plane, and a high-contrast ommatidial structure appeared (Figure 1E). However, the central part of the eye lost its contrast because of less illumination than in the peripheral regions of the curved eye surface. This issue was resolved by lifting the ring illuminator approximately 2° upward to the eye position. This improved optical alignment—called low-angle ring illumination stereomicroscopy (LARIS)—did not generate any glare and equally illuminated all parts of the eye, thereby providing a high-contrast image of the entire eye (Figure 1F). In this approach, the position of the ring light source is only slightly above the sample in the LARIS alignment so that the camera receives only the scattered light from the curved surface of the eye. This makes the top surface of each ommatidium slightly brighter than its basal areas, which creates high contrast between neighboring ommatidia and allows distinct visualization of each ommatidium (Figure 1F).
The LARIS method provides better contrast of ommatidial structure, orientation, and pigmentation status than what is obtained by conventional imaging methods of the Drosophila compound eye. A comparative analysis of different approaches used in eye imaging of Drosophila is given in Table 1. Here, we provide a detailed protocol for the LARIS method to image compound eyes of adult flies or other insects.
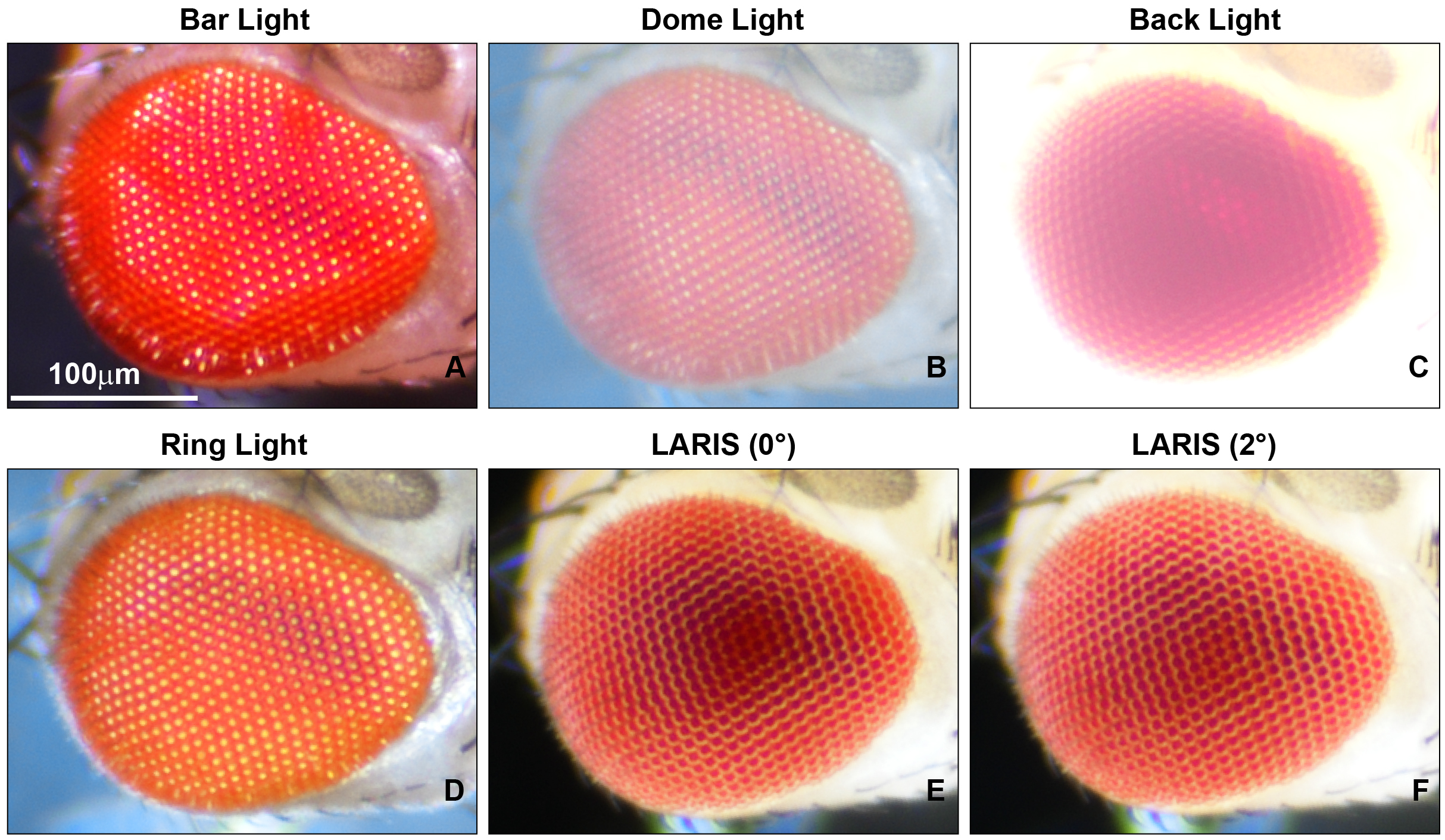
Figure 1. Microphotographs of an adult Drosophila compound eye under a simple stereomicroscope with different illumination methods. (A) bar light, (B) dome light, (C) back light, (D) ring light, (E) low-angle ring illumination stereomicroscopy (LARIS) (0°), and (F) LARIS (2°), as mentioned above each panel. All panels are the same sample imaged sequentially at the same magnification without remounting. Scale bar, 100 μm.
Table 1. Comparison of LARIS with other imaging methods for the adult Drosophila compound eye
| Features | Conventional | SEM | Nail polish | LARIS |
|---|---|---|---|---|
| Contrast | Low | Very high | High | High |
| Pigmentation record | Yes | No | No | Yes |
| Sample processing | None | Yes | Yes | None |
| Cost effectiveness | Low | Very high | Low | Low |
| Assay timing | Low | Very high | High | Low |
| Skills and resources | Low | High | Low | Low |
Materials and reagents
Biological materials
1. Adult flies (3 days old) of desired genotypes; we used GMR-GAL4/+; +/+, GMR-GAL4/+; UAS-HttQ138-mRFP/+, and GMR-GAL4/+; UAS-FUSWT/+ lines for adult eye imaging in this study.
Laboratory supplies
1. Glass slide (Blue Star microslide, 75 mm × 25 mm)
2. Transparent nail polish (Shade-40)
3. Fine forceps (Dumont Tweezer, catalog number: 72701-D)
4. Dissection needle (Syringes 31G)
5. Round synthetic paintbrush (Round paintbrush Size 4)
6. Ether (SRL, catalog number: 25049)
7. Etherizer (Tarson, catalog numbers: 630020 and 500043)
Equipment
1. Stereomicroscope (Nikon, model: SMZ800N)
2. Ring illuminator (AmScope LED-144B-ZK Black 144 PCS Adjustable LED Ring Light)
3. Microscope camera (Nikon, model: DS-Fi3)
Software and datasets
1. NIS, Elements Imaging Software, Basic Research (BR)
2. Fiji/ImageJ software (NIH, USA) (Java 6, available at imagej.nih.gov/ij)
Procedure
文章信息
稿件历史记录
提交日期: Oct 6, 2025
接收日期: Dec 28, 2025
在线发布日期: Jan 13, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Biswas, J., Kumar, A. and Singh, A. K. (2026). Low Angle Ring Illumination Stereomicroscopy (LARIS) Method for High-Contrast Imaging of Drosophila Compound Eyes. Bio-protocol 16(3): e5590. DOI: 10.21769/BioProtoc.5590.
分类
神经科学 > 感觉和运动系统 > 视觉系统
生物物理学 > 显微技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












