- EN - English
- CN - 中文
Turbo-RIP: A Protocol for TurboID-based RNA Immunopurification to Map RNA Landscapes in Plant Biomolecular Condensates
Turbo-RIP:基于 TurboID 的 RNA 免疫纯化方法,用于绘制植物生物分子凝聚体中的 RNA 图谱
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5587 浏览次数: 331
评审: Anonymous reviewer(s)
Abstract
Biomolecular condensates organize cellular processes through liquid–liquid phase separation, creating membrane-less compartments enriched in specific proteins and RNAs. Understanding their RNA composition is essential for elucidating plant stress responses, yet capturing these transiently associated RNAs remains technically challenging. We present Turbo-RIP (TurboID-based proximity labeling with RNA immunopurification), a comprehensive protocol for identifying condensate-associated RNAs in plants. Turbo-RIP employs the biotin ligase TurboID to label proximal proteins at 22 °C, followed by formaldehyde crosslinking and streptavidin-based capture of protein–RNA complexes. We provide detailed procedures for three cloning strategies, transformation of Nicotiana benthamiana and Arabidopsis thaliana, validation of TurboID activity, and RNA recovery. The protocol successfully captured processing body–associated RNAs with minimal background. Turbo-RIP enables systematic mapping of RNA populations within plant condensates under diverse conditions. The protocol requires 3–5 days from sample preparation to RNA isolation, with construct validation taking 2–4 weeks. All procedures use standard laboratory equipment, making Turbo-RIP accessible for plant molecular biology laboratories.
Key features
• Apply TurboID-based proximity labeling specifically for RNA capture in plant condensates.
• Optimize experimental conditions for different plant species and condensate types.
• Implement quality control measures and data analysis pipelines.
Keywords: TurboID (TurboID)Graphical overview
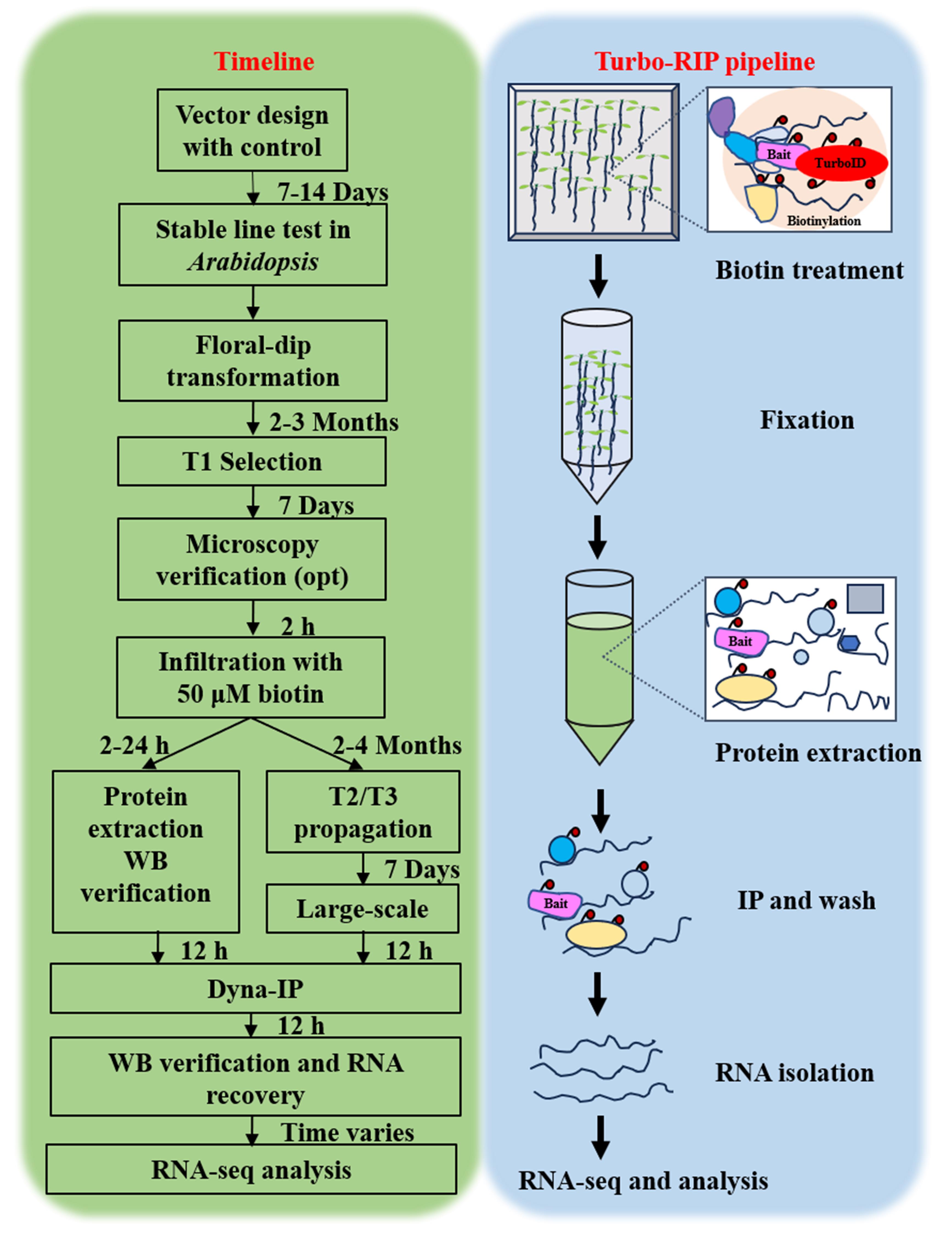
Turbo-RIP. T, transformation. Opt, optional. WB, western blot. IP, immunopurification. Dyna-IP, DynabeadsTM MyOne Streptavidin C1 immunopurification.
Background
Biomolecular condensates are dynamic assemblies formed through liquid–liquid phase separation (LLPS), creating specialized cellular microenvironments without membrane boundaries [1,2]. These structures compartmentalize specific proteins, RNAs, and metabolites, enabling rapid cellular responses to environmental changes. In plants, condensates regulate diverse processes including RNA processing, stress signaling, and metabolic channeling [3,4].
Traditional approaches for studying condensate composition face significant limitations. Crosslinking and immunoprecipitation (CLIP) methods often require highly specific antibodies and fail to capture the transient interactions characteristic of condensates [5]. The tandem RNA isolation procedure (TRIP) requires stable expression of RNA and binding proteins, which can be time-consuming. Hence, using TRIP, weak or highly dynamic interactions may be lost during a stringent purification [6,7]. Overall, the dynamic exchange of components and weak multivalent interactions that drive phase separation make compositional analysis particularly challenging.
Here, we present Turbo-RIP (TurboID-based proximity labeling with RNA immunopurification), a robust protocol that combines proximity-dependent biotinylation with RNA capture techniques. The method leverages TurboID, an engineered Escherichia coli biotin ligase that overcomes the temperature limitations of earlier BioID variants, functioning efficiently at plant-compatible temperatures (22 °C) [8]. TurboID catalyzes the formation of reactive biotin-AMP, which covalently modifies lysine residues on proteins within ~10 nm radius [9]. By capturing biotinylated RNA-binding proteins and their associated RNAs, Turbo-RIP enables comprehensive mapping of condensate RNA landscapes. The overall protocol is a modification of the APEAL approach (Tandemly Coupled Affinity Purification with Proximity-dEpendent LigAtion), which we have detailed previously [10,11].
Materials and reagents
Biological materials
1. Transient expression system: healthy Nicotiana benthamiana plants, 3–5 weeks old
2. Arabidopsis thaliana Columbia-0 (Col-0, five-six weeks) for construction of transgenic lines: TurboID-tagged protein of interest-expressing lines (protein of interest-TurboID) and TurboID-expressing lines (control line) [8]; select 8–10 independent lines via antibiotic plates and pick two lines of each for western blot (WB) verification and further experiments [12]. Five-day-old seedlings of transgenic lines are used for biotin incubation and other tests
3. Bacterial strain NEB® 10-beta Competent Escherichia coli (New England Biolabs, catalog number: C3019H) DH10B, genotype: Δ(ara-leu) 7697 araD139 fhuA ΔlacX74 galK16 galE15 e14- ϕ80dlacZΔM15 recA1 relA1 endA1 nupG rpsL (StrR) rph spoT1 Δ(mrr-hsdRMS-mcrBC)
4. Electrocompetent Agrobacterium (Agrobacterium tumefaciens) strain C58C1 RifR (pMP90) or GV3101 RifR
Reagents
Note: Reagents are kept at room temperature (RT) or other conditions according to the manufacturer's instructions. Reactions are performed according to the manufacturer's instructions unless otherwise specified.
1. LB broth/LB agar (Duchefa Biochemie, catalog numbers: L1704 and L1706)
2. MS plant medium (Murashige and Skoog medium) (Duchefa Biochemie, catalog number: M0221)
3. Gelrite (Duchefa Biochemie, catalog number: G1101)
4. Nuclease-free water (Sigma-Aldrich, catalog number: W4502)
5. 10 mM dNTP (Thermo Fisher Scientific, catalog number: 18427088)
6. Biotin (Sigma-Aldrich, catalog number: 2031), store at 4 °C, protect from light
7. 4′-Hydroxy-3′,5′-dimethoxyacetophenone, acetyl syringone (acetosyringone) (Sigma-Aldrich, catalog number: D134406), store at RT, protect from light
8. 2-(N-morpholino) ethanesulfonic acid (MES) (Sigma-Aldrich, catalog number: 4432-31-9)
9. 37% (w/v) formaldehyde (Sigma-Aldrich, catalog number: 1.04002.1000)
10. Glycine (Sigma-Aldrich, catalog number: 1.04201.0100)
11. Tri (hydroxymethyl) aminomethane (Tris) (Sigma-Aldrich, catalog number: 1.08387)
12. Ethylenediamine tetraacetic acid disodium salt (EDTA) (Sigma-Aldrich, catalog number: 6381-92-6)
13. Sodium chloride (NaCl) (Thermo Fisher Scientific, catalog number: S271-10)
14. Magnesium chloride (MgCl2) (VWR, catalog number: MK5958-04)
15. Calcium chloride (CaCl2) (VWR, catalog number: MK5958-04)
16. Sodium dodecyl sulfate (SDS) (MilliporeSigma, catalog number: L3771-1KG)
17. Triton X-100 (MilliporeSigma, catalog number: T9284-500 mL)
18. Glycerol (Sigma‐Aldrich, catalog number: G5516)
19. Dithiothreitol (DTT) (Thermo Fisher Scientific, catalog number: BP172-5)
20. Phenylmethylsulphonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: 195381)
21. Natrium azide (Na-Azide) (Merk, catalog number: S28222 917)
22. Diethyl pyrocarbonate (DEPC) (Sigma-Aldrich, catalog number: 016K3726)
23. Ethanol (EtOH) (Sigma-Aldrich, catalog number: 1.00974.2511)
24. Methanol (MeOH) (Millipore, catalog number: 34966-4 L)
25. Chloroform (Sigma-Aldrich, catalog number: 1.02444.1000)
26. TurboID plasmids and the corresponding codon-optimized sequence for plant-specific expression can be found in [8]; the original plasmids can be obtained from Addgene (https://www.addgene.org/search/catalog/plasmids/?q=turboid)
27. PhusionTM High‐Fidelity DNA Polymerase & dNTP mix or equivalent proofreading polymerase (Thermo Fisher Scientific, catalog number: F530S)
28. In Fusion® HD Cloning kit (Clonetech/Takara, catalog number: 638909)
29. GatewayTM BP ClonaseTM II enzyme mix (Thermo Fisher Scientific, catalog number: 11789-020)
30. GatewayTM LR ClonaseTM II enzyme mix (Thermo Fisher Scientific, catalog number: 11791020)
31. BsaI–HF Type IIS restriction enzyme optimized for Golden Gate Assembly (New England BioLab, catalog number: 10156743)
32. T4 DNA ligase (New England BioLab, catalog number: M0202S)
33. pENTRTM/D−TOPO® Cloning kit, with One Shot® TOP10 Chemically Competent E. coli (Thermo Fisher Scientific, catalog number: ab178021)
34. Primary vectors: pDONR221 (GatewayTM) and pGWB501/601 [8] or similar
35. Rifampicin (Duchefa Biochemie, catalog number: R0146.0005)
36. Kanamycin (Duchefa Biochemie, catalog number: K0126.0005)
37. Hygromycin B (Duchefa Biochemie, catalog number: H0192)
38. Spectinomycin (Duchefa Biochemie, catalog number: S0188.0005)
39. GeneJET Gel Extraction kit (Thermo Fisher Scientific, catalog number: K0692)
40. GeneJET Plasmid Miniprep kit (Thermo Fisher Scientific, catalog number: K0503)
41. DynabeadsTM MyOne Streptavidin C1 (Thermo Fisher Scientific, catalog number: 65601), store at 4 °C, ≥2,800 pmol free biotin/mg beads
42. DynaMagTM‐2 magnet (Thermo Fisher Scientific, catalog number: 112321D)
43. PD‐10 columns (Cytiva, catalog number: 17085101)
44. Protease inhibitor cocktail (Sigma‐Aldrich, catalog number: P9599)
45. RNase inhibitor (40 U/μL) (Thermo Fisher Scientific, catalog number: EO0381)
46. Proteinase K (Thermo Fisher Scientific, catalog number: 25530049, 20 mg/mL), store at -20 °C
47. Phenol-chloroform-isoamyl alcohol mixture (25:24:1) pH 4.0–5.0 (VWR, catalog number: 136112-00-0)
48. Isopropanol (2-propanol) (VWR, catalog number: BDH1133-4LP)
49. β-Mercaptoethanol (Sigma‐Aldrich, catalog number: 444203)
50. Sodium acetate (NaAc) (Sigma‐Aldrich, catalog number: S8750)
51. GlycoBlue (Thermo Fisher Scientific, catalog number: AM951, 15 mg/mL), nuclease and protease-free
52. Polyoxyethylene (20) sorbitan monolaurate (Tween 20) (Sigma-Aldrich, catalog number: 655204)
53. IGEPAL (CA‐630) (Sigma‐Aldrich, catalog number: MK5958-04)
54. 4 x Laemmli buffer (Bio‐Rad, catalog number: 1610747)
55. 30% acrylamide/Bis solution (29.2:0.8) (Bio-Rad, catalog number: 1610156)
56. Ammonium persulfate (APS) (Sigma-Aldrich, catalog number: A3678)
57. N,N,N,N′-Tetramethyl-ethylenediamine (TEMED) (Sigma-Aldrich, catalog number: T7024)
58. Any kDTM Mini-PROTEAN® TGXTM Precast Protein Gels, 10-well format or similar (Bio-Rad, catalog number: 4569033)
59. Bovine serum albumin fraction V (BSA) (Sigma-Aldrich, catalog number: 9048-46-8)
60. PageRulerTM Plus Prestained Protein Ladder or similar (Thermo Fisher Scientific, catalog number: 26619)
61. Polyvinylidene fluoride membrane (PVDF), 0.22 μm, 30 × 3 M (Bio‐Rad, catalog number: 1620177 or similar)
62. Phosphate-buffered saline (PBS) tablet (Sigma‐Aldrich, catalog number: P4417)
63. 3′,3″,5′,5″-tetrabromophenolsulfonphthalein (Bromophenol blue) (Sigma-Aldrich, catalog number: B5525)
64. Streptavidin-horseradish peroxidase (Strep-HRP) (Sigma-Aldrich, catalog number: GERPN1231 or similar), use in dilution of 1:25,000 in 3% BSA with 1× PBST
65. Mouse anti‐FLAG‐horseradish peroxidase (FLAG-HRP) (Sigma-Aldrich, catalog number: A8592 or similar), use in dilution 1:25,000 in 3% BSA with 1× PBST
66. Rat anti‐Tubulin (Santa Cruz Biotechnology, 1:1,000)
67. Anti‐rat [IRDye® 800 CW Goat α‐Rat IgG (H + L), LI‐COR, 925‐32219, 1:10,000]
68. Enhanced chemiluminescence (ECL) HRP substrate of regular sensitivity, typically as a two-component system consisting of a stable peroxide solution and an enhanced luminol solution (Cytiva or similar)
69. ECL of high sensitivity: SuperSignalTM West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, catalog number: 34094 or similar)
70. RNase-free DNase (e.g., RQ1 from Promega); RQ1 RNase-Free DNase is provided with the following reagents: 10× RQ1 RNase-free DNase buffer, RQ1 RNase-free DNase, and RQ1 DNase stop solution
71. 100 μM oligo d(T) and/or 50 μM random hexamers and/or specific primers against known RNA
72. Qubit® RNA HS Assay kit (Thermo Fisher Scientific, catalog number: Q32852)
73. NEBNext® UltraTM II RNA Library Prep with sample purification beads (New England BioLab, catalog number: E7775S)
74. NEBNext Multiplex Oligos for Illumina (Dual Index Primers Set 1) (New England BioNordika BioLab, catalog number: E7600S)
75. RiboMinusTM Plant kit (Thermo Fisher Scientific, catalog number: A1083808)
76. High-performance reverse transcriptase (Thermo Fisher Scientific, catalog number: 18090200); SuperScript IV is provided with the following reagents: 5× SSIV buffer, 0.1 M DTT, and SuperScript IV reverse transcriptase
77. qRT-PCR quantification master mix (e.g., Thermo Fisher Scientific, catalog number: 11813923)
78. RNaseZap® RNase decontamination solution (ThermoFisher Scientific, catalog number: AM9780)
79. Agilent DNA 7500 kit (Agilent, catalog number: 5067-1506)
80. Appropriate primer set to detect the RNA/cDNA of interest as well as negative and positive controls if possible [e.g., EBF1f (5′-3′): TCAGATCTTTAGTTTTGCCGGTGA; EBF1r (5′-3′): TGCTACTTACAAGCGTAAGCCA]
Solutions
Note: Prepare all solutions using ultrapure water (DNase-, RNase-, and protease-free, obtained by purifying deionized water to achieve a resistivity of 18 MΩ·cm at 25 °C) or another specified solvent, using analytical-grade reagents. Store all reagents at room temperature unless otherwise indicated. Exercise general safety precautions when handling hazardous chemicals: wear gloves, work in a fume hood when necessary, and strictly follow all regulations for waste disposal.
1. Diethyl pyrocarbonate (DEPC) water (see Recipes)
2. 1.5 M Tris-HCl, pH 8.8 (see Recipes)
3. 0.5 M Tris-HCl, pH 6.8 (see Recipes)
4. 1 M Tris-HCl, pH 8.0 (see Recipes)
5. 1 M Tris-HCl, pH 7.5 (see Recipes)
6. LB medium (see Recipes)
7. Half-strength (1/2) MS plant medium (see Recipes)
8. 50% glycerol (see Recipes)
9. 1 M MgCl2 (see Recipes)
10. 1 M CaCl2 (see Recipes)
11. 5 M NaCl (see Recipes)
12. 0.5 M EDTA, pH 8.0 (see Recipes)
13. 1 M DTT (see Recipes)
14. 100 mM acetosyringone (see Recipes)
15. 50 mM biotin (see Recipes)
16. 0.5 M MES (see Recipes)
17. Infiltration buffer (see Recipes)
18. Kanamycin 100 mg/mL (see Recipes)
19. Spectinomycin 50 mg/mL (see Recipes)
20. Rifampicin 25 mg/mL (see Recipes)
21. 2 M glycine (see Recipes)
22. 20% (v/v) Triton X-100 (see Recipes)
23. Extraction buffer (EB) (see Recipes)
24. Wash buffer (see Recipes)
25. Dilution buffer (see Recipes)
26. Elution buffer (see Recipes)
27. 10% (w/v) SDS (see Recipes)
28. 10% (w/v) APS (see Recipes)
29. 10× SDS running buffer (see Recipes)
30. 1× SDS transfer buffer (see Recipes)
31. 10× PBS (see Recipes)
32. 3% (w/v) BSA blocking solution (see Recipes)
33. 0.1% (w/v) Ponceau staining solution (see Recipes)
34. Protease K buffer (see Recipes)
35. Homogenization buffer (see Recipes)
36. 3 M NaAc (see Recipes)
Recipes
Note: We followed the manufacturer’s recommendations to adjust the pH of solutions at 4 °C. The procedure presented in this protocol is mainly performed at cold temperatures or on ice to prevent RNA or protein degradation, so the pH of solutions must be adjusted at 4 °C as the working temperature for the protocol. The volume of all solutions is adjusted with DEPC water unless specifically indicated.
1. DEPC water
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DEPC | 0.1% | 1 mL |
| Total | n/a | 1,000 mL |
Stir overnight and autoclave. Store at RT.
2. 1.5 M Tris-HCl, pH 8.8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-HCl | 1.5 M | 181.7 g |
| Total | n/a | 1,000 mL |
Adjust pH with 6 N HCl, autoclave, and store at 4 °C.
3. 0.5 M Tris-HCl, pH 6.8
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-HCl | 0.5 M | 60.6 g |
| Total | n/a | 1,000 mL |
Adjust pH with 6 N HCl, autoclave, and store at 4 °C.
4. 1 M Tris-HCl, pH 8.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-HCl | 1 M | 121.1 g |
| Total | n/a | 1,000 mL |
Adjust pH with 6 N HCl, autoclave, and store at 4 °C.
5. 1 M Tris-HCl, pH 7.5
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris-HCl | 1 M | 121.1 g |
| Total | n/a | 1,000 mL |
Adjust pH with 6 N HCl, autoclave, and store at 4 °C.
6. LB medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Peptone | 1% (w/v) | 10 g |
| Yeast extract | 0.5% (w/v) | 5 g |
| NaCl | 1% (w/v) | 10 g |
| Agar | 1.2% (w/v) | 12 g |
| Total | n/a | 1,000 mL |
Autoclave and store at RT.
7. Half-strength (1/2) MS plant medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MS | 2.15 g/L | 2.15 g |
| Sucrose | 1% (w/v) | 10 g |
| Gelrite | 1% (w/v) | 10 g |
| Total | n/a | 1,000 mL |
Use 1 M KOH to adjust pH to 5.8, add gelrite, autoclave, and store at 4 °C.
8. 50% glycerol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glycerol | 50% (v/v) | 50 mL |
| Total | n/a | 100 mL |
Autoclave and store at RT. Alternatively, filter-sterilize using a 0.22 μm filter and store at RT.
9. 1 M MgCl2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MgCl2·6H2O | 1 M | 20.33 g |
| Total | n/a | 100 mL |
Autoclave and store at RT.
10. 1 M CaCl2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CaCl2·2H2O | 1 M | 14.702 g |
| Total | n/a | 100 mL |
Autoclave and store at RT.
11. 5 M NaCl
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | 5 M | 29.22 g |
| Total | n/a | 100 mL |
Autoclave and store at RT.
12. 0.5 M EDTA, pH 8.0
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| EDTA | 0.5 M | 14.621 g |
| Total | n/a | 100 mL |
Use 10 N NaOH to adjust pH to 8.0.
13. 1 M DTT
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DTT | 1 M | 1.54 g |
| Total | n/a | 10 mL |
Dissolve in 10 mL of ddH2O and aliquot into 1.5 mL tubes stored at -20 °C. Do not thaw and refreeze aliquots.
14. 100 mM acetosyringone
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetylsyringone | 100 mM | 0.3924 g |
| Total | n/a | 20 mL |
Dissolve in 20 mL of DMSO and aliquot into 1.5 mL tubes stored at -20 °C. Do not thaw and refreeze aliquots.
15. 50 mM biotin
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Biotin | 50 mM | 0.12 g |
| Total | n/a | 10 mL |
Dissolve in 10 mL of DMSO and aliquot into 1.5 mL tubes stored at -20 °C. Do not thaw and refreeze aliquots.
16. 0.5 M MES
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| MES | 0.5 M | 9.762 g |
| Total | n/a | 100 mL |
Use 10 N NaOH to adjust pH to 5.7. Store at RT.
17. Infiltration buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 0.5 M MES (Recipe 16) | 10 mM | 2 mL |
| 1 M MgCl2 (Recipe 9) | 10 mM | 1 mL |
| Total | n/a | 100 mL |
Prepare just before use. The solution is not stable if made in advance.
18. Kanamycin 100 mg/mL
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Kanamycin | 100 mg/mL | 1 g |
| Total | n/a | 10 mL |
Weigh 1 g of kanamycin and dissolve in 9 mL of ddH2O in a 15 mL Falcon tube. Add ddH2O to a total volume of 10 mL, dissolve completely, pass through a 0.22 μm filter, and aliquot it. The stock may be kept at -20 °C for up to 1 year. Use 50 μg/mL as a working concentration.
19. Spectinomycin 50 mg/mL
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Spectinomycin | 50 mg/mL | 0.5 g |
| Total | n/a | 10 mL |
Weigh 0.5 g of spectinomycin and dissolve in 9 mL of ddH2O in a 15 mL Falcon tube. Add ddH2O to a total volume of 10 mL, dissolve completely, pass through a 0.22 μm filter, and aliquot it. The stock may be kept at -20 °C for up to 1 year. Use 50 μg/mL as a working concentration.
20. Rifampicin 25 mg/mL
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Rifampicin | 25 mg/mL | 0.25 g |
| Total | n/a | 10 mL |
Weigh 0.25 g of rifampicin and dissolve in 9 mL of DMSO in a 15 mL Falcon tube. Add DMSO to a total volume of 10 mL, dissolve completely, and aliquot it. The stock may be kept at -20 °C for up to 1 year. Use 50 μg/mL as a working concentration.
21. 2 M glycine
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Glycine | 2 M | 150 g |
| Total | n/a | 1,000 mL |
Autoclave and store at RT.
22. 20% (v/v) Triton X-100
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100 | 20% (v/v) | 10 mL |
| Total | n/a | 50 mL |
Store at RT.
23. Extraction buffer (EB)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl pH 7.5 (Recipe 5) | 50 mM | 500 μL |
| 5 M NaCl (Recipe 11) | 150 mM | 333.3 μL |
| 1 M MgCl2 (Recipe 9) | 2.5 mM | 25 μL |
| 50% glycerol (Recipe 8) | 10% (v/v) | 2 mL |
| 20% Triton X-100 (Recipe 23) | 0.5% (v/v) | 250 μL |
| 0.1 M PMSF | 1 mM | 100 μL |
| Protease inhibitor cocktail (100×) | 1% | 100 μL |
| RNase inhibitor | 20 U/mL | 5 μL |
| Total | n/a | 10 mL |
Prepare just before use. The solution is not stable if made in advance.
24. Wash buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl pH 7.5 (Recipe 5) | 20 mM | 200 μL |
| 5 M NaCl (Recipe 11) | 150 mM | 333.3 μL |
| 1 M MgCl2 (Recipe 9) | 2.5 mM | 25 μL |
| 50% glycerol (Recipe 8) | 10% (v/v) | 2 mL |
| 20% Triton X-100 (Recipe 23) | 0.2% (v/v) | 100 μL |
| 1 M DTT (Recipe 13) | 0.5 mM | 5 μL |
| Protease inhibitor cocktail (100×) | 1% | 10 μL |
| RNase inhibitor | 20 U/mL | 5 μL |
| Total | n/a | 10 mL |
Prepare just before use. The solution is not stable if made in advance.
25. Dilution buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl pH 7.5 (Recipe 5) | 20 mM | 200 μL |
| 5 M NaCl (Recipe 11) | 150 mM | 333.3 μL |
| 1 M MgCl2 (Recipe 9) | 2.5 mM | 25 μL |
| 50% glycerol (Recipe 8) | 10% (v/v) | 2 mL |
| Protease inhibitor cocktail (100×) | 1% | 100 μL |
| RNase inhibitor | 20 U/mL | 5 μL |
| Total | n/a | 10 mL |
Prepare just before use. The solution is not stable if made in advance.
26. Elution buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 4× Laemmli buffer | 2× | 1 mL |
| 50 mM biotin (Recipe 15) | 5 mM | 200 μL |
| 10% (w/v) SDS (Recipe 29) | 2% (w/v) | 400 μL |
| 1 M DTT (Recipe 13) | 100 mM | 200 μL |
| ddH2O | n/a | 200 μL |
| Total | n/a | 2 mL |
Prepare just before use. The solution is not stable if made in advance.
27. 10% (w/v) SDS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| SDS | 10% | 10 g |
| Total | n/a | 100 mL |
Store at RT.
Caution: SDS is a fine powder. It should be weighed under a fume hood to avoid inhalation or use a fume mask.
28. 10% APS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| APS | 10% (w/v) | 1 g |
| Total | n/a | 10 mL |
Dissolve in 10 mL of ddH2O and aliquot into 1.5 mL tubes stored at -20 °C. Do not thaw and refreeze aliquots.
29. 10× SDS running buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base | 0.2501 M | 30.3 g |
| Glycine | 1.924 M | 144.4 g |
| SDS | 1% (w/v) | 10 g |
| Total | n/a | 1,000 mL |
Prepare in advance and store at RT. Stable for 1 year.
30. 1× SDS transfer buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tris base | 0.02501 M | 3.03 g |
| Glycine | 0.1924 M | 14.44 g |
| Methanol | 20% | 200 mL |
| Total | n/a | 1,000 mL |
Prepare just before use.
31. 10× PBS
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | n/a | 80.1 g |
| Na2HPO4 | n/a | 14.4 g |
| KCl | n/a | 2 g |
| KH2PO4 | n/a | 2.7 g |
| Total | n/a | 1,000 mL |
Use 6 N HCl to adjust pH to 7.4. Autoclave and store at RT.
32. 3% BSA blocking solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| BSA | 3% (w/v) | 3 g |
| PBST | n/a | 100 mL |
Prepare just before use. The solution is not stable if made in advance.
33. 0.1% (w/v) Ponceau staining solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ponceau S | 0.1% (w/v) | 0.1 g |
| Glacial acetic | 5% (w/v) | 5 mL |
| Total | n/a | 100 mL |
Prepare in advance. The prepared Ponceau solution should be stored in the dark at 4 °C for one year.
34. Protease K buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl pH 8.0 (Recipe 4) | 30 mM | 30 μL |
| Total | n/a | 1 mL |
Prepare just before use.
35. Homogenization buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 1 M Tris-HCl pH 8.0 (Recipe 4) | 100 mM | 1,000 μL |
| 5 M NaCl (Recipe 11) | 100 mM | 200 μL |
| 0.5 M EDTA (Recipe 12) | 0.5 mM | 10 μL |
| 1 M MgCl2 (Recipe 9) | 2.5 mM | 25 μL |
| 0.1 M PMSF | 1 mM | 100 μL |
| β-mercaptoethanol | 1% | 100 μL |
| Total | n/a | 10 mL |
Prepare just before use. The solution is not stable if made in advance.
36. 3 M NaAc
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaAc | 3 M | 24.6102 g |
| Total | n/a | 100 mL |
Use 10 N NaOH to adjust pH to 5.2. Store at RT.
Laboratory supplies
Note: Clean the laboratory supplies with RNaseZap® RNase decontamination solution or use supplies indicated with DNase and RNase-free.
1. Pipette set and tips (Eppendorf or similar)
2. Miracloth (Merck Millipore, catalog number: 475855)
3. Mesh (SefarNitex, catalog number: 03-100/44)
4. 1 mL disposable syringe
5. Mortar
6. Spatula
7. Gloves
8. 1.5 mL Protein LoBind microcentrifuge tubes (Eppendorf, catalog number: 022431081)
9. 15- or 50-mL polypropylene Falcon tubes or equivalent (DNase and RNase-free)
10. PCR tubes and caps (VWR, catalog number: 20170-010)
11. Syringe filter, 0.2 μm (Thermo Fisher Scientific, catalog number: 725-2520)
12. 1 mm gap sterile electroporation cuvette compatible with all common electroporators
13. Soil (Hasselfors P-soil or equivalent) mixed with sand (Rada sand) at a 5:1 (v/v) ratio: fill pots with the mix (e.g., pots of size 10 × 10 × 8 cm)
14. Reach-in or walk-in growth chambers at 22 °C during subjective daytime and 19 °C subjective night, with a cycle of 14/10 h light/dark (or 16/8 h light/dark). The light intensity at the leaf surface should be around 150 μE/m2/s from white light plant growth lamps. Pot watering schedule depends on the plant growth stage, avoiding flooding and extreme drought conditions (normally, 2–3 days/per time before flowering, 1–2 days/per time after flowering). Relative humidity can be kept constant and uniform at 50%–60%. For example, Arabidopsis thaliana and Nicotiana benthamiana plants can be grown in Aralab or Percival growth chambers.
Equipment
Note: Clean the laboratory equipment with RNaseZap® RNase decontamination solution for all steps involving RNA.
1. Vertical laminar flow clean bench
2. Incubator shaker that can reach 28 °C and 37 °C
3. Thermomixer (Eppendorf, model: 5382)
4. Ice bath
5. Microwave
6. Refrigerator (4 °C)
7. Freezer (-20 °C)
8. Ultra-low temperature freezer (-80 °C)
9. 37 °C and 42 °C water baths
10. Spectrophotometer
11. Ultraviolet gel imaging system (Azure Biosystems, model: c600)
12. NanoDrop Ultra UV-Vis spectrophotometer (Thermo Fisher Scientific, catalog number: NDULTRAGL)
13. Bio-Rad Gene Pulser X cell total electroporation system or similar
14. Table centrifuge (Eppendorf, model: centrifuge 5418, for RT)
15. Table centrifuge (Eppendorf, model: centrifuge 5417R, for 4 °C and -20 °C)
16. PCR machine (Bio-Rad, Veriti 96 wells, catalog number: 4375786)
17. Autoclave (Spire Integrated Solutions, model: PRIMUS)
18. Agarose gel electrophoresis equipment (Bio-Rad, model: PowerPacTM Basic Power Supply, Horizontal Electrophoresis Systems)
19. Mini PROTEAN® 3 System glass plates for casting in-house-made gels
20. Protein electrophoresis apparatus (e.g., Bio-Rad or equivalent)
21. Luminescent image analyzer
22. Odyssey infrared imaging system or similar
23. Real-time PCR system (e.g., LightCycler 480 System from Roche)
24. Vacuum pump (Techtum, model: MZ 2 NT)
25. Optional for sequencing: fluorimetric dsDNA quantification system (e.g., Qubit, Thermo Fisher)
26. Optional for sequencing: fragment analyzer (e.g., BioAnalyzer, Agilent)
27. Optional for sequencing: high-throughput sequencing device (e.g., Illumina) with corresponding library preparation kit
Software and datasets
1. Construct and cloning design software (e.g., SnapGene 7.2 or similar)
2. R software (4.3.3) package with Bioconductor
3. Galaxy (https://usegalaxy.org)
4. Cytoscape v. 3.5.1 (https://cytoscape.org/)
Procedure
文章信息
稿件历史记录
提交日期: Nov 10, 2025
接收日期: Dec 26, 2025
在线发布日期: Jan 8, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Zhang, Z., Xu, Y., Liu, H., Liu, C. and Moschou, P. N. (2026). Turbo-RIP: A Protocol for TurboID-based RNA Immunopurification to Map RNA Landscapes in Plant Biomolecular Condensates. Bio-protocol 16(3): e5587. DOI: 10.21769/BioProtoc.5587.
分类
植物科学 > 植物分子生物学 > RNA > RNA-蛋白质相互作用
分子生物学 > RNA > RNA 纯化 > 亲和纯化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












