- EN - English
- CN - 中文
The Generation of Tissue-Specific ECM Hydrogels From Melanoma and Associated Organs to Study Cancer Biology
利用黑色素瘤及相关器官来源的组织特异性细胞外基质水凝胶研究肿瘤生物学
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5586 浏览次数: 112
评审: Dharma PallyPrudhvi Chand MallepaddiAnonymous reviewer(s)
Abstract
The extracellular matrix (ECM) critically shapes melanoma progression and therapeutic response, yet commonly used matrices such as Matrigel fail to capture tissue- and disease-specific ECM properties. This protocol provides a streamlined and scalable method for generating murine, tissue-specific ECM hydrogels from skin, lung, and melanoma tumors, therefore overcoming the restricted materials of mouse-derived ECM. The workflow integrates tissue-tailored decellularization, lyophilization, mechanical fragmentation, pepsin digestion, and physiological polymerization to produce hydrogels that reliably preserve fibrillar collagen architecture and organ-specific ECM cues. Decellularization efficiency and ECM integrity are validated by DNA quantification, H&E staining, and Picrosirius Red staining analysis. These hydrogels provide a species- and tissue-matched platform for studying melanoma–ECM–immune interactions, pre-metastatic niche features, and therapy-induced ECM remodeling. Overall, this protocol offers a reproducible and physiologically relevant ECM model that expands experimental capabilities for melanoma biology and treatment-resistance research and that can be easily extended to other tumors and tissues.
Key features
• A miniaturized, tissue-specific workflow for generating ECM hydrogels from small murine skin, lung, and melanoma tissues, overcoming size limitations of existing protocols.
• Preservation of native ECM architecture using tailored decellularization steps validated by DNA quantification, H&E, and Picrosirius Red staining.
• A standardized digestion–gelation process optimized for heterogeneous and lipid-rich murine tissues, enabling reproducible hydrogel formation at defined ECM concentrations.
• A physiologically relevant platform capturing melanoma- and organ-specific ECM cues for studying ECM–tumor–immune interactions and therapy-induced remodeling.
Keywords: Extracellular matrix (ECM) (细胞外基质(ECM))Graphical overview
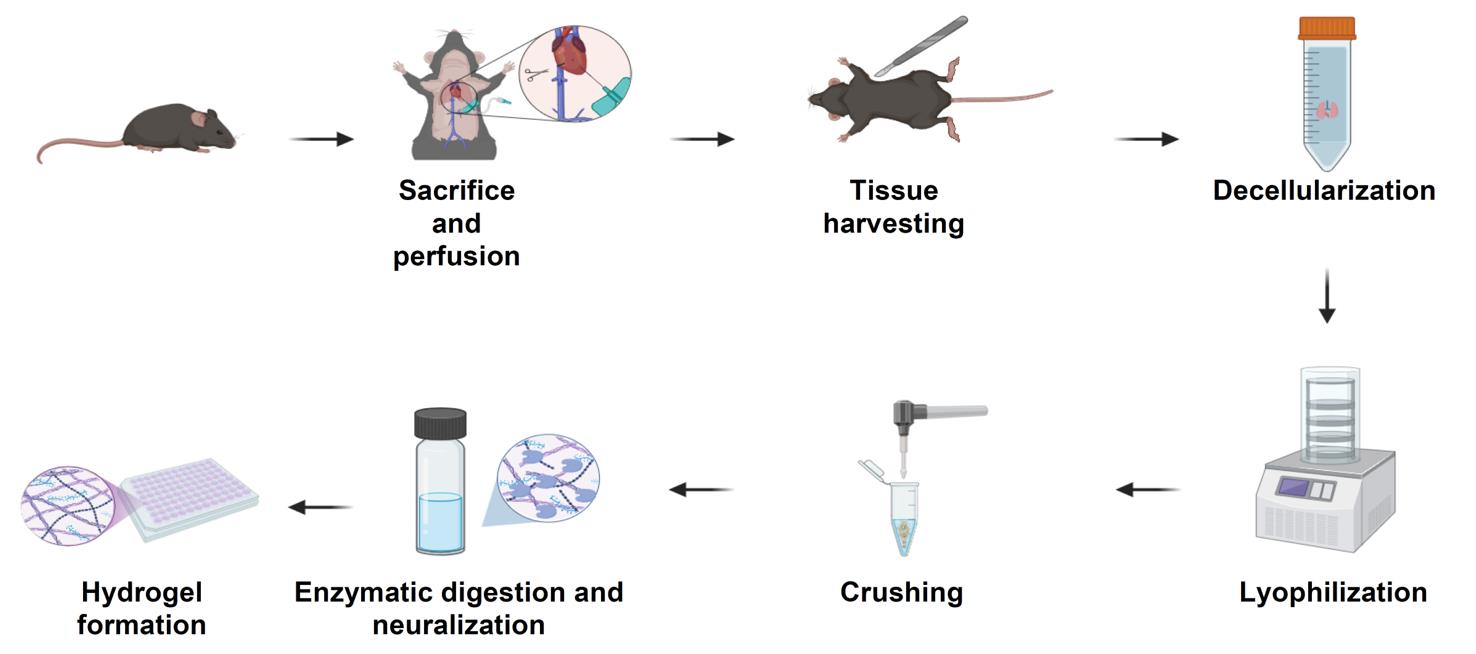
Schematic overview of the extracellular matrix (ECM) hydrogel preparation protocol. Following sacrifice and perfusion of mice, tissues of interest are harvested and subjected to a decellularization process to remove cellular material (24–48 h, depending on tissue type). Decellularized tissues are lyophilized for 24 h (lungs and tumors) or 48 h (skin), then mechanically crushed into a fine powder. The ECM powder is enzymatically digested under acidic conditions for 24 h (skin) or 48 h (lungs and tumors), neutralized, and adjusted to physiological ionic strength. The resulting pre-gel solution is incubated at 37 °C to form stable ECM hydrogels suitable for downstream biological applications.
Background
Melanoma, the most lethal form of skin cancer, arises from transformed melanocytes and is characterized by high metastatic potential and marked immunogenicity [1]. Despite its substantial tumor mutational burden and responsiveness to immune checkpoint inhibitors, up to two-thirds of patients fail to benefit or eventually relapse following initial responses, highlighting the knowledge gaps in treatment resistance and disease progression [2].
Recent studies suggest that ECM remodeling in melanoma, among other cancers, plays a central role in invasion, metastasis, and immune regulation. Alterations such as increased matrix stiffness, collagen fiber alignment, and crosslinking, mediated by enzymes such as lysyl oxidases (LOX) and matrix metalloproteinases (MMPs), promote cancer cell dissemination and can physically hinder immune cell infiltration [3]. These changes not only facilitate melanoma progression but also contribute to phenotype switching and resistance to targeted or immune-based therapies. Preclinical studies targeting ECM components, such as integrins, hyaluronan, and MMPs, have demonstrated potential synergy with immunotherapy, pointing to the ECM as a promising therapeutic co-target in melanoma [4]. Therefore, the advancement of new tools to study ECM in melanoma can advance melanoma research and serves as a basis for the development of new treatments for melanoma.
The composition and organization of ECM proteins vary across tissues, reflecting their unique properties and functional requirements [5]. Additionally, the ECM undergoes dynamic changes over time, adapting to tissue needs and the embedded cells within each organ [6]. In cancer, the ECM plays a pivotal role, usually supporting tumor development and progression. Unlike normal tissues, the ECM of solid tumors exhibits significant alterations in protein composition and structure [7]. Changes in the ECM occur not only in the primary tumor but also in distant organs [8]. ECM remodeling in distant organs creates a supportive environment for disseminated cancer cells, creating a pre-metastatic microenvironment, allowing cancer cells to home to such sites and colonize there. These ECM alterations are driven by factors secreted by the primary tumor, such as extracellular vesicles and LOX, and involve interactions among resident stromal cells and recruited immune cells [9].
Current in vitro ECM models, including collagen gels, Matrigel, and synthetic hydrogels, do not reflect the diverse ECM compositions of melanoma across anatomical or metastatic sites [10,11]. While tissue-derived hydrogels generated from human or porcine organs provide more physiological ECM environments [12], they are limited by availability, batch variability, and lack of compatibility with murine experimental systems. Mouse-derived ECM hydrogels would enable species-matched studies, facilitate integration with in vivo perturbations, and support mechanistic work in genetically engineered or syngeneic models. However, their use has been restricted by the small size of murine tissues, which often yields insufficient material for conventional decellularization and hydrogel preparation [13].
This protocol addresses these limitations by establishing a miniaturized, tissue-specific workflow for generating ECM hydrogels from a small quantity of murine skin, lung, and melanoma tumors. The method preserves native ECM cues and supports downstream applications for modeling melanoma–ECM–immune interactions, metastatic niche conditions, and therapy-induced ECM remodeling [14,15]. Beyond melanoma, the same workflow can be adapted to other solid tumor types; we have successfully applied similar decellularization steps to LLC, 4T1, and PyMT tumors, indicating broad applicability across murine cancer models.
Materials and reagents
Biological materials
1. YUMM 1.7 murine melanoma cells (ATCC, CRL-3362)
2. B16-F10 murine melanoma cells (ATCC, CRL-6475)
3. C57BL/6 mice (Harlan Laboratories Israel)
Reagents
1. Double-distilled water (DDW)
2. 1× phosphate-buffered saline (PBS) (Sigma-Aldrich, catalog number: L0615-500ML)
3. 10× PBS (Sigma-Aldrich, catalog number: D1408-500ML)
4. Triton X-100 (Sigma-Aldrich, catalog number: T8787)
5. Ammonium hydroxide solution 25% (Biolab, catalog number: 125050100)
6. DNase I powder 3,520 U/mg (Worthington, catalog number: LS002139)
7. Ethanol, absolute >99.8% (Gadot Group, catalog number: 64-17-5)
8. Hydrogen peroxide (H2O2), 33% (Panreac, catalog number: 131077.1211)
9. Acetic acid glacial 99.7% (BioLab, catalog number: 107052100)
10. Tris base (Sigma-Aldrich, catalog number: T1503)
11. Ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, catalog number: EDS-100G)
12. Trypsin 0.25%–EDTA (0.038%) in HBSS (IMBH, catalog number: L0931-500ML)
13. Pepsin from porcine gastric mucosa powder (Sigma-Aldrich, catalog number: P7012-1G)
14. Sodium hydroxide pearls (NaOH) (Biolab, catalog number: 1908059100)
15. Hydrochloric acid 32% (HCl) (BioLab, catalog number: 846050100)
Solutions
1. Triton X-100/ammonium hydroxide solution (see Recipes)
2. Triton X-100/0.26% EDTA/0.69% Tris (see Recipes)
3. 0.4% peracetic acid/4% ethanol solution (see Recipes)
4. Pepsin solution (see Recipes)
5. DNase I solution 50 U/mL (see Recipes)
6. 70% ethanol (see Recipes)
7. 3% H2O2 (see Recipes)
Recipes
1. Triton X-100/ammonium hydroxide solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100 | 1% | 10 mL |
| 25% ammonium hydroxide | 0.004% | 4 mL |
| DDW | n/a | 986 mL |
2. Triton X-100/0.26% EDTA/0.69% Tris
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100 | 1% | 10 mL |
| EDTA powder | 0.26% | 2.6 g |
| Tris base | 0.69% | 6.9 g |
| DDW | n/a | 990 mL |
3. 0.4% peracetic acid/4% ethanol solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Acetic acid glacial | 0.4% | 1 mL |
| 33% H2O2 | 0.03% | 1 mL |
| Ethanol, absolute | 4% | 40 mL |
| DDW | n/a | 958 mL |
4. Pepsin solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Pepsin from porcine gastric mucosa | 1 mg/mL | 5 mg |
| 0.01 M HCl | 0.01 M | 5 mL |
Note: This recipe is calculated based on preparing solutions for processing 50 mg of dry ECM powder. Volumes and reagent quantities should be scaled accordingly if processing larger or smaller tissue amounts. Ensure precise weighing of the ECM powder prior to preparation and adjust all components proportionally to maintain the specified final concentrations.
5. DNase I solution 50 U/mL
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DNase I powder 3,520 U/mg | 50 U/mL | 0.071 mg |
| 1× PBS | n/a | 5 mL |
6. 70% ethanol
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Ethanol, absolute >99.8% | 70% | 350 mL |
| DDW | n/a | 150 mL |
7. 3% H2O2
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 33% H2O2 | 3% | 45.5 mL |
| DDW | n/a | 454.5 mL |
Note: All recipes should be made fresh.
Laboratory supplies
1. Eppendorf tubes 1.7 mL (Corning Inc., catalog number: 28324032)
2. Scintillation vials (Sigma-Aldrich, catalog number: 986546)
3. Depilatory cream
4. 27G needle (Bactlab, catalog number: BD300635)
5. Scissors (Sigma-Aldrich, catalog number: 41122408)
6. Butterfly needle (Bar Naor, catalog number: BNSCLPV23g)
7. 20 mL syringe (BD Emerald, catalog number: 307736)
8. 200 μL pipette tips (TH Geyer, catalog number: 7695884)
9. 50 mL conical tubes (Greiner Bio-One, catalog number: 227261)
Equipment
1. Lyophilizer (Labogene, model: CoolSafe 110-4, catalog number: 7001000115)
2. Shaker-incubator (Benchmark, catalog number: H2010)
3. Horizontal shaker (ELMI, catalog number: S-3.02)
4. Cellular centrifuge (Heraeus, catalog number: PICO17)
5. Intelli-Mixer (ELMI, catalog number: RM-2L)
6. Handheld tissue homogenizer (VISOSCI, catalog number: B0CG4KPQW5)
7. 37 °C dry incubator (Heracell, catalog number: 150i)
8. -80 °C freezer
9. Refrigerator (2–8 °C)
Procedure
文章信息
稿件历史记录
提交日期: Oct 17, 2025
接收日期: Dec 22, 2025
在线发布日期: Jan 8, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Mogilevsky, Y., Sharon-Yagol, C., Manobla, B., Saad, S., Raviv, Z. and Shaked, Y. (2026). The Generation of Tissue-Specific ECM Hydrogels From Melanoma and Associated Organs to Study Cancer Biology. Bio-protocol 16(3): e5586. DOI: 10.21769/BioProtoc.5586.
分类
癌症生物学 > 通用技术 > 肿瘤微环境
生物物理学 > 生物工程 > 医用生物材料
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











