- EN - English
- CN - 中文
A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice
适用于清醒自由活动小鼠的低应激、长时稳定尾静脉置管及精准给药方案
(*contributed equally to this work) 发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5585 浏览次数: 105
评审: Anonymous reviewer(s)

相关实验方案

从食物到酒精的狂饮:雄性Wistar大鼠嗜酒行为之间的顺序相互作用
Sergio Cuesta-Martínez [...] Cruz Miguel Cendán
2023年08月05日 1345 阅读
Abstract
Tail vein catheterization in mice is a standard technique for precise drug delivery in pharmacological research, offering high accuracy and reproducibility. However, existing techniques face significant limitations in maintaining long-term stable catheter patency in awake, freely moving mice, and there is currently no standardized, detailed protocol for tail vein catheterization. Current methods suffer from high rates of catheter dislodgement, increased animal stress from repeated injections, and movement restrictions, all of which introduce confounding variables in behavioral and pharmacological studies. We have developed a simple and efficient fixation method that maintains stable tail vein catheter patency for more than 60 min while allowing complete freedom of movement. This protocol employs a strain relief loop design and multi-point fixation strategy, effectively preventing catheter dislodgement during extended periods while minimizing animal stress. This protocol has been successfully applied across multiple research areas, including metabolic studies, behavioral assessments, and neuropharmacological research in awake mice, achieving >95% catheter retention with normal animal behavior, providing a reliable technical platform for long-term awake-state research applications.
Key features
• Maintains catheter stability for over 60 min in freely moving awake mice without physical restraint.
• Catheter placement accommodates natural mouse behaviors (grooming, walking, standing).
• Compatible with swivel systems for continuous drug infusion during behavioral testing.
• Applicable to diverse research applications (metabolic studies, behavioral assessments, neuropharmacological research).
Keywords: Mouse tail vein catheterization (小鼠尾静脉置管)Graphical overview
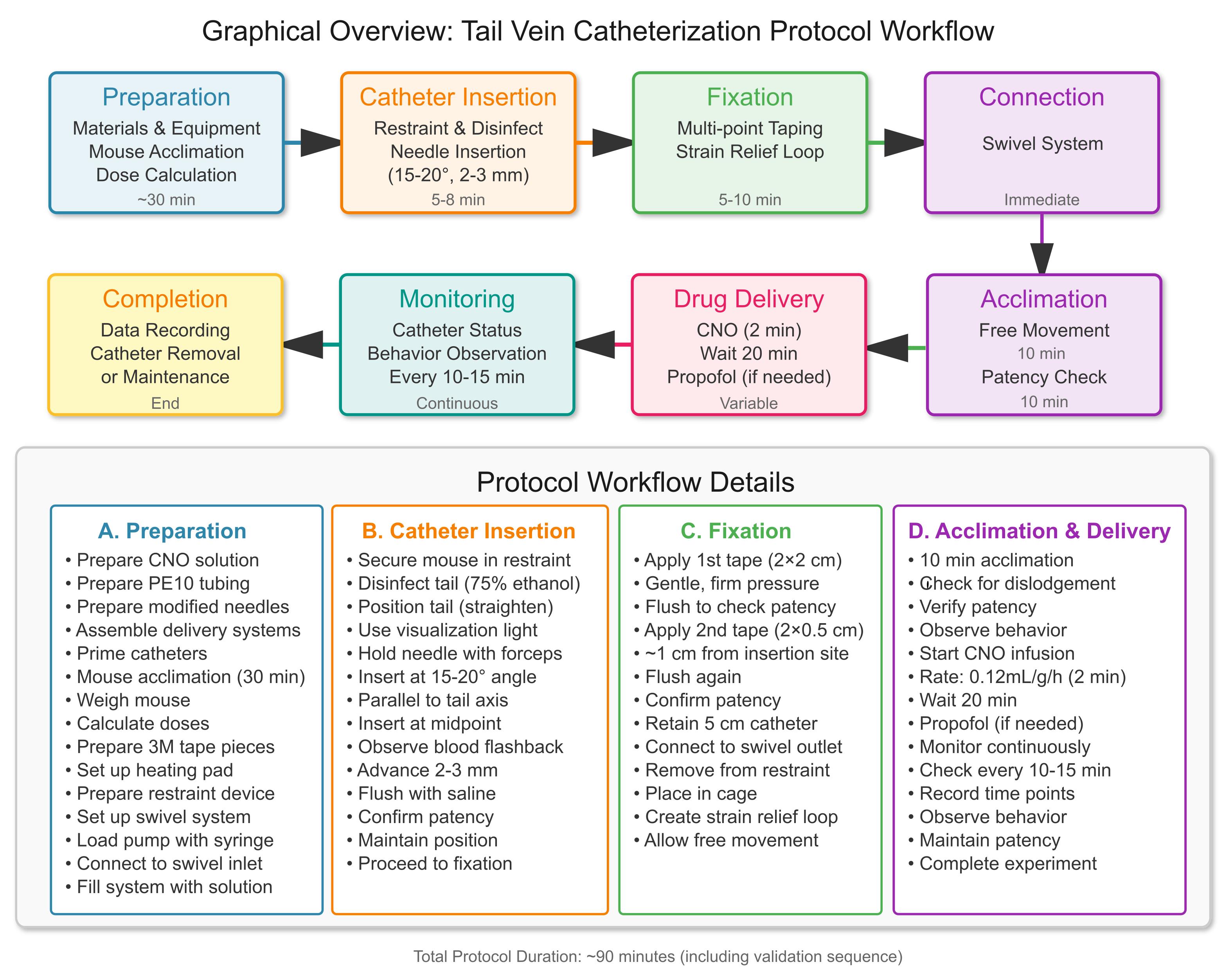
Tail vein catheterization protocol workflow
Background
Tail vein catheterization in mice is a fundamental technique in pharmacological research, providing precise and reproducible drug delivery for studying drug effects, pharmacokinetics, and pharmacodynamics [1,2]. This method is particularly valuable in neuropharmacological studies, where maintaining stable plasma drug concentrations is crucial for understanding drug mechanisms and therapeutic effects [3,4]. However, traditional tail vein injection methods face significant limitations when applied to long-term studies requiring continuous drug administration, particularly in awake, freely moving animals [5,6], due to technical challenges in catheterization, inadequate fixation methods, and long-duration limitations. Current tail vein catheterization methods primarily rely on single injections [6] or short-term catheter placement under anesthesia, which are insufficient for studies requiring extended drug exposure in conscious animals [7]. Due to technical challenges associated with tail vein catheterization, many researchers have adopted alternative approaches, such as intraperitoneal injection instead of intravenous delivery [8] or single bolus injection instead of continuous stable drug administration [7]. While these alternatives may be technically easier to implement, they introduce significant limitations: intraperitoneal injection exhibits slower and unpredictable drug absorption compared to intravenous delivery [8,9], and a single injection cannot maintain the stable plasma drug concentrations required for pharmacokinetic studies and chronic treatment protocols [8,9]. Repeated injections, while providing some temporal control, introduce significant animal stress that may confound behavioral and physiological measurements [10,11]. Short-term catheterization techniques typically maintain patency for less than 10 min in awake animals, which is insufficient for most experimental protocols requiring extended observation periods [12]. Collectively, these commonly used approaches are suboptimal for experiments requiring sustained, precisely controlled intravenous exposure in awake animals, because they either fail to maintain stable plasma drug concentrations over extended periods (bolus or non-IV routes) or introduce restraint- and handling-related stress (restraint-based infusion or repeated injections). Therefore, there remains a need for a low-stress method that supports continuous tail vein infusion for ≥60 min in awake, freely moving mice.
The technical challenges of long-term tail vein catheterization in awake mice stem from several factors. First, the small size and delicate nature of mouse tail veins make them prone to catheter dislodgement during normal animal movements such as grooming, walking, and standing [12]. Second, traditional fixation methods typically require pharmacological restraint, which introduces stress variables that affect experimental outcomes and animal welfare [13,14]. Third, there is currently no standardized protocol that effectively addresses both the issue of catheter stability during extended periods and animal comfort [11,15].
Developing a reliable long-term tail vein catheterization protocol for awake mice would significantly advance research capabilities across multiple fields [16]. Such a protocol would enable continuous drug delivery during behavioral testing, allow maintenance of stable plasma drug concentrations, and minimize animal stress by maintaining natural movement patterns. This is particularly important for diverse research applications, including neuropharmacological research, metabolic studies, behavioral assessments, and neuroimaging studies, where the effects of drugs on behavior, cognition, and neural function need to be evaluated in awake animals without the confounding effects of anesthesia or restraint stress.
Materials and reagents
Biological materials
1. Mice, 6–18 weeks of age, body weight 20–35 g (Beijing Vital River Laboratory Animal Technology Co., Ltd.)
Reagents
1. Sterile normal saline (0.9% NaCl) (Beyond, catalog number: ST341-500ml or equivalent)
2. Experimental drugs (as needed, such as CNO, propofol, etc.)
3. Clozapine N-oxide (CNO) [Sigma-Aldrich, catalog number: C0832; ≥98% (HPLC), or equivalent]
4. Propofol injection (10 mg/mL) (Fresenius Kabi Deutschi and GmbH Hafnerstraβe 36, A-8055 Graz, Austria, or equivalent)
5. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418) for preparing drug solutions
6. 75% ethanol (Sigma-Aldrich, catalog number E7023 or equivalent) for disinfection
Solutions
1. CNO working solution (see Recipes)
Recipes
1. CNO working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CNO | 0.075 mg/mL | 0.3 mg |
| DMSO | 0.25% | 10 μL |
| Normal saline | 0.9% | 3,990 μL |
| Total | - | 4,000 μL |
Note: CNO working solution is used for i.v. infusion. Prepare a CNO stock solution by dissolving 0.3 mg of CNO in 10 μL of DMSO and store protected from light at -20 °C. Prepare the working solution by diluting the stock solution with 3,990 μL of sterile normal saline to a final volume of 4,000 μL. The final CNO concentration is 0.075 mg/mL, and the final DMSO concentration is 0.25% (v/v) (10 μL of DMSO in 4,000 μL of total volume). CNO is light-sensitive; therefore, prepare and handle solutions with minimal light exposure. The working solution is best prepared fresh; if not used immediately, store at 4 °C protected from light and use within 48 h. Freezing the working solution and repeated freeze–thaw cycles are not recommended due to potential loss of stability.
Laboratory supplies
1. Mouse restraint device (for tail vein procedures); dimensions: approximately 120 × 120 × 60 mm, inner diameter ~35 mm, with an adjustable tail access port (JiShuo, model: ZK-XGD-2, or equivalent)
2. PE10 polyethylene tubing, 0.28 mm inner diameter, 0.61 mm outer diameter (Scientific Commodities, Inc., catalog number: BB31695-PE/8)
3. 3M paper tape (for catheter fixation)
4. 30 G BD PrecisionGlide needles (Becton Dickinson, catalog number: 305106, 30G × 1/2” or equivalent)
5. Fine forceps (ophthalmic forceps) (RWD, catalog number: F12006-10) for holding needles during tail vein puncture
6. 1 mL syringes (Beyotime, catalog number: FS801-30pcs or equivalent)
7. Digital balance (for weighing mice)
8. Heating pad (for maintaining mouse body temperature)
Equipment
1. Microinjection pump (any precision infusion pump)
2. 30GA single-channel plastic swivel with welded 30 G needle (for continuous infusion during free movement)
Procedure
文章信息
稿件历史记录
提交日期: Nov 1, 2025
接收日期: Dec 29, 2025
在线发布日期: Jan 13, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ye, Y., Fu, X., Wang, J. and Fang, J. (2026). A Low-Stress, Long-Duration Stable Tail Vein Catheterization and Precise Drug Delivery Protocol for Awake, Freely Moving Mice. Bio-protocol 16(3): e5585. DOI: 10.21769/BioProtoc.5585.
分类
神经科学 > 行为神经科学 > 实验动物模型
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










