- EN - English
- CN - 中文
Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids
快速简便构建分化型子宫内膜悬浮类器官的模型
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5584 浏览次数: 145
评审: Anonymous reviewer(s)
Abstract
Nowadays, the use of 3D cultures (organoids) is considered a valuable experimental tool to model physiological and pathological conditions of organs and tissues. Organoids, retaining cellular heterogeneity with the presence of stem, progenitor, and differentiated cells, allow the faithful in vitro reproduction of structures resembling the original tissue. In this context, the growth of endometrial organoids allows the generation of 3D cultures characterized by a hollow lumen, secretory activity, and apicobasal polarity and displaying phenotypical modification in response to hormone stimulation. However, a limitation in currently used models is the absence of stromal cells in their structure; as a result, they miss epithelial–stromal interactions, which are crucial in endometrial physiology. We developed a novel 3D model to generate endometrial organoids grown in floating MatrigelTM droplets in the presence of standard culture medium. From a structural point of view, these novel floating 3D cultures develop as gland-like structures constituted by epithelial cells organized around a central lumen and retain the expression of endometrial and decidual genes, like previously published organoids, although with a phenotype resembling hormonally differentiated structures. Importantly, floating organoids retain stromal cells which grow in close contact with the epithelial cells, localized within the internal or external portion of the organoid structure. In summary, we present a simple and rapid model for generating 3D endometrial organoids that preserve epithelial–stromal cell interactions, promoting the formation of differentiated organoids and enabling the study of reciprocal modulation between epithelium and stroma.
Key features
• Development of 3D endometrial organoids using 10% FBS-DMEM/F12 complete medium in less than a month.
• Preservation of stromal and epithelial cell populations, favoring the study of their interaction.
• Development of differentiated endometrial organoids without the need for in vitro hormonal stimulation.
Keywords: Endometrial organoids (子宫内膜类器官)Graphical overview
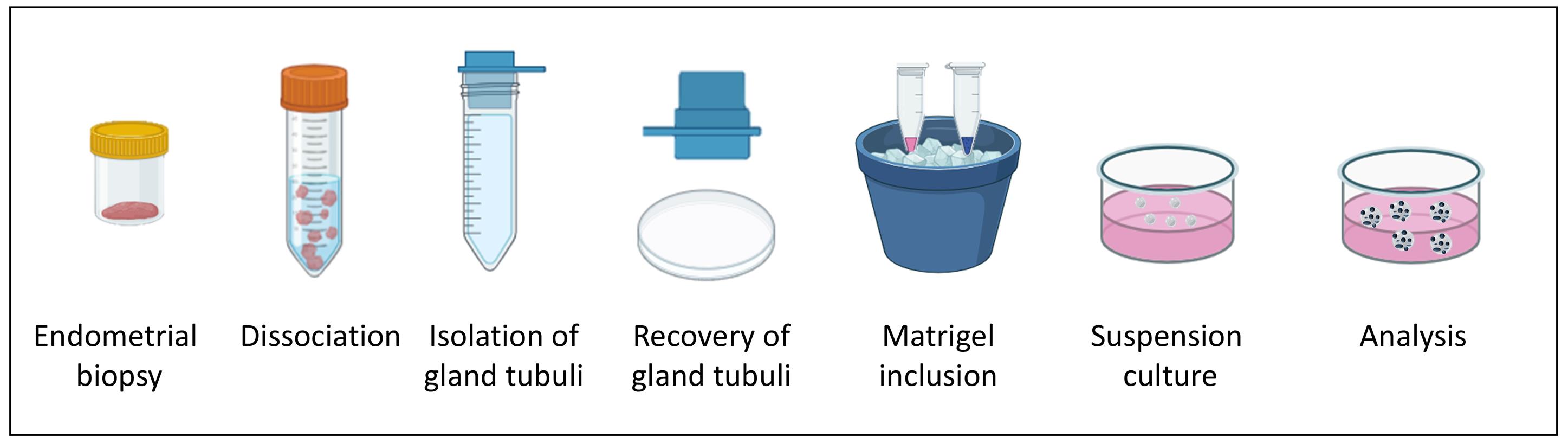
Workflow of endometrial floating organoid formation
Background
Three-dimensional (3D) cell culture models represent a new frontier in biomedical research, dramatically changing the experimental approaches to tissue physiology and pathology. In particular, gland organoids are considered highly relevant in vitro models, since they can reconstitute the complex interactions among different cell types, which are at the basis of the tissue physiology and are often altered in pathological conditions.
In the past years, different types of epithelial organoids have been developed to study mechanisms determining reproductive tract diseases, providing an impressive advancement in modeling the reproductive system. Epithelial organoids have been established from many different regions of the female reproductive tract; among others, endometrial organoids represent nowadays a pillar in the study of endometrial dysfunctions and infertility [1]. In particular, endometrial organoids, reproducing endometrial architecture in vitro, provide an extremely valuable tool to model different physiological conditions and analyze tissue alterations occurring in pathology, as well as the efficacy of pharmacological treatments. In fact, they can recreate 3D structures from tissues derived from both healthy donors and endometrial-related disease patients.
Several different approaches have been used to develop organoid models able to reproduce endometrial complexity [2,3]. The endometrial tissue used to develop organoids can be obtained by laparoscopy or biopsy catheter, from all stages of the menstrual cycle, from the decidua, atrophic tissues, or menstrual blood [4–7], and from both fresh and cryopreserved samples [8]. Several types of pathological tissues (e.g., carcinomas, endometriotic lesions, or biopsies from infertile women) can also easily generate organoids, thus representing novel models to study endometrial physiology and pathology in different biological conditions, including all the phases of the implantation process [4,5,9].
Two seminal reports were published in 2017, in which the generation of endometrial gland organoids was described [5,7]. Organoids in both studies were generated starting from the in vitro growth of isolated glandular fragments, which were then embedded in an artificial extracellular matrix (i.e., MatrigelTM) in chemically defined culture media. 3D cultures displayed a genetically stable phenotype and self-renewing and self-organizing abilities, preserved responsiveness to hormone exposure, and exhibited cellular differentiation upon specific hormonal stimulation. Published endometrial organoids are organized as a single columnar epithelium with a central lumen, and are suitable for long-term cultures when grown in the presence of activators of Wnt and ERK1/2 signaling and inhibited TGFβ and BMP pathways [10,11].
Although endometrial gland organoids recapitulate the features of uterine glands, preserving spontaneously organized in vitro luminal and glandular elements, they cannot reproduce the midluteal endometrium, since they lack stromal and immune cell components and are devoid of vascular elements. This limits the information obtained on epithelial–stromal interactions, and thus their suitability for many highly specific analyses in which all these cell populations play a significant role.
To overcome these issues (mainly the lack of stromal cells within the 3D culture), more complex culture models have been developed and named assembloids. These were established by incorporating stromal cells with endometrial glandular organoids after their generation [3,12]. However, this approach is technically and experimentally challenging, namely regarding the introduction of a luminal epithelium and/or uterine natural killer cells and the development of protocols for long-term maintenance and propagation. A more advanced model of assembloids has been reported, in which endometrial epithelial and stromal cells were cultured using a matrix and air–liquid interface [13]. This model seems to better recapitulate endometrium anatomy, different cell population composition, and hormone-induced changes along the endometrial menstrual cycle. However, this model is technically challenging and difficult to develop.
In this scenario, we report a novel, simple, and cost-effective endometrial organoid model based on floating MatrigelTM droplets, which allows the formation of organoids containing both epithelial and stromal cellular components.
Starting from glandular elements isolated from fresh or cryopreserved Pipelle biopsies from women who underwent assisted reproductive technologies, we generated free-floating MatrigelTM organoids grown in 10% fetal bovine serum (FBS)-DMEM/F12, which we named “floating organoids,” self-organized as round or elongated structures, formed by epithelial cells integrated with stromal cells around a central lumen. Floating organoids were obtained from 100% of the biopsies analyzed [14], demonstrating a high reproducibility even with very small amounts of starting tissue or when it is derived from patients.
The comparison of this novel model with conventional organoids evidenced a morphology similar to the conventional organoid, being formed by a monolayer of epithelial cells organized as round or elongated sprouted structures surrounding an empty cavity. As observed in conventional organoids, floating organoids express E-cadherin, mucin 1, and acetyl-α-tubulin, confirmed by the epithelial origin of the cells composing the glandular structure. A significant presence of vimentin-expressing stromal cells, mostly localized in close contact with epithelial cells, was detected in floating but not in conventional organoids.
In our simplified model, we propose the use of conventional medium supplemented only with FBS, representing an easy and inexpensive way to obtain 3D cultures. Compared to the use of chemically defined media, FBS provides a more suitable environment for the growth of stromal cells, which is prevented in conventional organoid cultures by a medium that may actually counteract stromal cell growth and persistence in the 3D structure. Moreover, the floating culture growth also prevents stromal cells from the organoids from growing on the plastic interface of the Petri dish, consequently favoring their integration with the endometrial epithelium. This is a particularly relevant difference between the two types of 3D cultures, since the reduced presence of stroma cells represents a major limitation of conventional organoids, as the interaction between epithelial and stromal cells plays a central role in hormonal differentiation. In fact, the persistent presence of stromal cells promotes cell differentiation, as assessed for decidualization marker expression, making floating organoids a valuable model of differentiated organoids. On the other hand, the differentiation of floating spheroids in serum-supplemented medium, without the necessity of hormonal differentiation, leads to a reduction in stemness, and the differentiation status reduces the possibility of prolonged in vitro propagation, being limited to one or two passages.
In conclusion, here we describe a simple and rapid protocol to generate differentiated endometrial organoids, which retain both epithelial and stromal cells in the 3D structure. The interaction between these cell populations may allow the study of their reciprocal modulation, in particular during the differentiation process.
Materials and reagents
Biological materials
1. Human endometrial tissues (0.5 × 1.0 cm) (generated in patients who underwent assisted reproductive technologies at the Reproductive Medicine Unit, Gynecology and Obstetrics Department of the International Evangelical Hospital, Genova, Italy); samples were collected during the secretory phase of the cycle, without hormonal stimulation, with a Pipelle catheter.
Reagents
1. Collagenase Type V (Sigma-Aldrich, catalog number: C9263-100MG)
2. Dispase II (Sigma-Aldrich, catalog number: D4693-1G)
3. DMEM/F12 (Euroclone, catalog number: ECM0095L)
4. Fetal bovine serum (FBS) (Gibco, catalog number: 10270106)
5. DNase I (Sigma-Aldrich, catalog number: DN25-100MG)
6. PBS 1× (Euroclone, catalog number: ECB4004L)
7. Antibiotics Pen/Strep 100× (Euroclone, catalog number: ECB 3001D)
8. L-Glutamine 200 mM (Euroclone, catalog number: ECB3000D)
9. Ethanol (Sigma-Aldrich, CAS number: 64-17-5)
10. Parafilm (AMCOR, catalog number: PM-996)
11. MatrigelTM growth factor reduced (GFR) (Corning, catalog number: 354230)
12. 16% formaldehyde solution (Thermo Scientific, catalog number: 28908)
13. 0.1 M glycine (Sigma-Aldrich, CAS number: 56-40-6)
14. Normal goat serum (Sigma-Aldrich, catalog number: G9023)
15. Triton X-100 (Sigma-Aldrich, CAS number, 9036-19-5)
16. Rabbit anti-vimentin (EPR3776) antibody (Abcam, catalog number: ab92547)
17. Mouse anti-cadherin (HECD-1) antibody (Abcam, catalog number: ab1416)
18. Mouse anti-acetyl-α-tubulin (Lys40) (6-11B-1) antibody (Cell Signaling, catalog number: 12152)
19. Goat anti-mouse IgG (H-L) Alexa FluorTM 568 antibody (Invitrogen, catalog number: A-11004)
20. Goat anti-rabbit IgG (H+L) Alexa FluorTM 647 antibody (Invitrogen, catalog number: A21245)
21. Sytox Blue nucleic acid stain (Invitrogen, catalog number: S11348)
22. Cell recovery solution (Corning, catalog number: 354253)
23. Aurum Total RNA mini kit (Bio-Rad, catalog number: 7326820)
24. Sodium acetate (Sigma-Aldrich, CAS number, 127-09-3)
25. Calcium acetate (Sigma-Aldrich, CAS number, 62-54-4)
26. Edu DetectPro Cell Proliferation Imaging kit (Baseclick GmbH, catalog number BCK-EduPro-IM488)
Solutions
1. Collagenase V stock solution 100× (see Recipes)
2. Dispase II stock solution 40× (see Recipes)
3. DNase I stock solution 100× (see Recipes)
4. DMEM/F12 basal medium (see Recipes)
5. 10% FBS-DMEM/F12 complete medium (see Recipes)
6. Enzyme digestion solution (see Recipes)
7. 4% Formaldehyde stock solution (see Recipes)
8. 1% Formaldehyde working solution (see Recipes)
9. Blocking-permeabilizing solution 1× (see Recipes)
Recipes
1. Collagenase V stock solution 100×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Collagenase V | 40 mg/mL | 100 mg |
| PBS w/o Ca2+ and Mg2+ | 1× | to 2.5 mL |
| Total | - | 2.5 mL |
Dissolve 100 mg of collagenase type V powder in 2.5 mL of PBS w/o Ca2+ and Mg2+ and sterilize through a 0.22 μm filter. Store aliquots at -20 °C.
2. Dispase II stock solution 40×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Dispase II | 50 U/mL | 1 g |
| 10 mM NaAc pH 7.5 and 5 mM CaAc | - | to 10 mL |
| Total | - | 10 mL |
Prepare a 20 mL solution of 10 mM NaAc (pH 7.5) with 5 mM CaAc and sterilize by filtration with a 0.22 μm filter. Dissolve 1 g of Dispase II in 10 mL of the filtered NaAc + CaAc solution.
3. DNase I stock solution 100×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DNase I | 40 mg/mL | 100 mg |
| H2O | - | to 2.5 mL |
| Total | - | 2.5 mL |
4. DMEM/F12 basal medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM/F12 | 1× | 490 mL |
| L-Glutamine | 2 mM | 5 mL |
| Pen/Strep | Pen 100 U/mL; Strep 0.1 mg/mL | 5 mL |
| Total | - | 500 mL |
5. 10% FBS-DMEM/F12 complete medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| DMEM/F12 basal medium | 1× | 450 mL |
| FBS | 10% | 50 mL |
| Total | - | 500 mL |
6. Enzyme digestion solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 10%FBS-DMEM/F12complete medium | 1× | 3,820 mL |
| Collagenase V 100× | 0.4 mg/mL | 40 μL |
| Dispase 40× | 1.25 U/mL | 100 μL |
| DNase 100× | 0.4 mg/mL | 40 μL |
| Total | - | 4 mL |
7. 4% formaldehyde stock solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 16% formaldehyde solution | 4% | 10 mL |
| PBS 1× | - | 30 mL |
| Total | - | 40 mL |
8. 1% formaldehyde working solution
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| 4% formaldehyde solution | 1% | 2.5 mL |
| PBS 1× | - | 7.5 mL |
| Total | - | 10 mL |
9. Blocking-permeabilizing solution 1×
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Normal goat serum | 10% | 0.5 mL |
| 1% Triton X-100 | 0.1% | 0.5 mL |
| PBS 1× | - | 4 mL |
| Total | - | 5 mL |
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 0030120086)
2. 15 and 50 mL conical tubes (Euroclone, catalog numbers: ET5015B, ET5050B)
3. 5 and 10 mL sterile pipettes (Euroclone, catalog numbers: EPS05N, EPS10N)
4. 10, 200, and 1,000 μL sterile tips (Euroclone, catalog numbers: ECTD00010, ECTD00200, ECTD01005)
5. 100 μm cell sieves (100 μm cell strainer) (Corning, catalog number: 431752)
6. 35, 60, and 100 mm Petri dishes (Euroclone, catalog numbers: ET2035, ET2060, ET2100)
7. Disposable stainless steel blade scalpels No. 23 (GIMA, catalog number: 27067)
8. Sterile tweezers
9. 3 mL plastic Pasteur pipette (Copan, catalog number: 200)
10. Empty 96-well rack of 10 μL tips (Eppendorf ep Dualfilter T.I.P.S., catalog number: 0030078500)
11. Nunc non-treated multidish 4 wells (Thermo Scientific, catalog number: 179820)
Equipment
1. Refrigerator 2–8 °C (Liebherr, model: SRFvg 3501-20A-001)
2. Freezer -20 °C (Liebherr, model: GP 2733 Index 20D/001) and -80 °C (Forma Scientific, model: 925)
3. Water bath (GFL)
4. Biological safety cabinet (BioAir Top-Safe, model: 1.8)
5. Laboratory centrifuge with rotor for 10- and 50-mL conical tubes (Eppendorf, model: 5810 R)
6. Laboratory centrifuge with rotor for 1.5- and 2.0-mL tubes (Eppendorf, model: 5415 R)
7. Water jacketed CO2 incubator (Forma Scientific Instruments, model: 3111)
8. Automated cell counter (Bio-Rad, model: TC20)
9. Digital inverted microscope (Leica Microsystems, model: DMIL) equipped with camera (Leica Microsystems, model: ICC50HD)
10. Confocal microscope (Leica Microsystems, model: Stellaris 8 STED)
Procedure
文章信息
稿件历史记录
提交日期: Nov 3, 2025
接收日期: Dec 17, 2025
在线发布日期: Jan 7, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bajetto, A., Pattarozzi, A., Corsaro, A., Tremonti, B. F., Thellung, S., Barbieri, F. and Florio, T. (2026). Simple and Rapid Model to Generate Differentiated Endometrial Floating Organoids. Bio-protocol 16(3): e5584. DOI: 10.21769/BioProtoc.5584.
分类
干细胞 > 类器官培养
细胞生物学 > 细胞分离和培养 > 3D细胞培养
发育生物学 > 细胞生长和命运决定 > 分化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














