- EN - English
- CN - 中文
Detailed Method for the Purification of Rhamnogalacturonan-I (RG-I) in Arabidopsis thaliana
拟南芥中鼠李半乳糖醛酸聚糖 I(RG-I)的纯化方法详述
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5579 浏览次数: 144
评审: Anonymous reviewer(s)
Abstract
The plant cell wall is a dynamic and complex extracellular matrix that not only provides structural integrity and determines cell shape but also mediates intercellular communication. Among its major components, pectins play essential roles in cell adhesion, wall porosity, hydration, and flexibility. Rhamnogalacturonan-I (RG-I), a structurally diverse pectic polysaccharide, remains one of the least understood components of the plant cell wall. Its backbone is substituted with arabinan, galactan, and arabinogalactan side chains that vary in length, branching, and composition across tissues, species, and developmental stages. In addition, RG-I can undergo modifications such as backbone acetylation, further contributing to its structural complexity and functional diversity. To advance understanding of RG-I, we present a detailed method for isolating RG-I from the model plant Arabidopsis thaliana. Leveraging Arabidopsis as a model system provides major advantages owing to its well-characterized genome and powerful molecular toolkit, enabling deeper investigation into the roles of RG-I in plant development and responses to environmental stress. Our method consists of two major steps: an initial chemical extraction using oxalate, followed by endo-polygalacturonase (EPG) digestion to fragment the pectic domains. An advantage of this approach is that it produces a dry material that can be stored at room temperature without special handling and does not introduce chemicals that may interfere with downstream analyses. The purified RG-I can be used for detailed compositional and structural analyses, as well as for functional studies of enzymes involved in pectin biosynthesis, modification, and degradation. Although this protocol was developed for isolating RG-I from Arabidopsis rosette leaves, it is also applicable to other Arabidopsis organs and other plant species.
Key features
• This protocol provides a detailed description of RG-I isolation from Arabidopsis rosette leaves.
• The isolated RG-I can be used for compositional and structural analyses and serves as a substrate for functional studies of enzymes.
• This protocol is also applicable for isolating RG-I from other Arabidopsis organs and from different plant species.
Keywords: Plant cell wall (植物细胞壁)Graphical overview
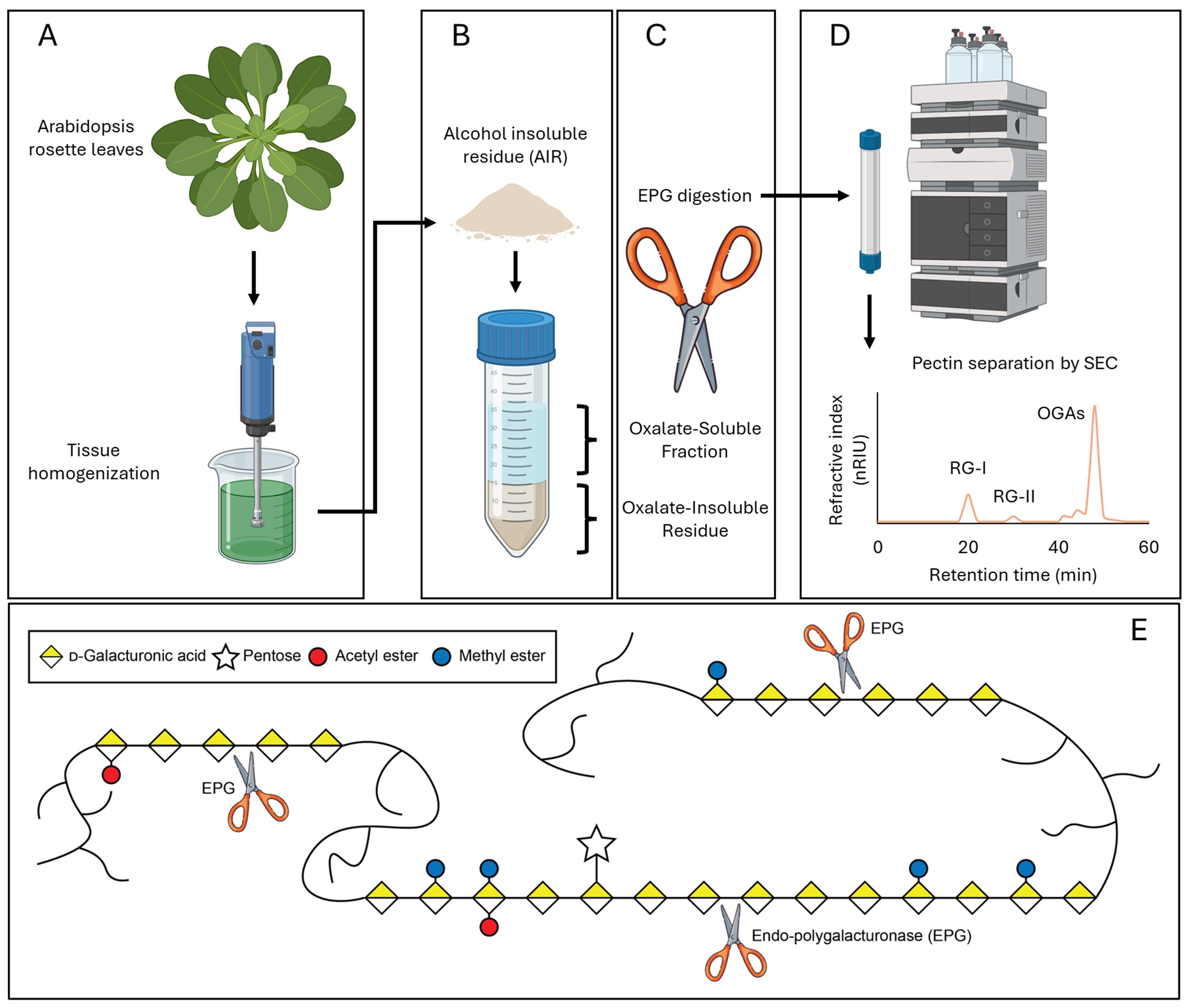
An outline of the steps for extracting rhamnogalacturonan-I (RG-I) from Arabidopsis rosette leaves. (A) Arabidopsis leaves are ground into a homogenate. (B) The resulting grounded plant material is treated to produce alcohol-insoluble residue (AIR), which is then treated with ammonium oxalate, resulting in an oxalate-soluble fraction and oxalate-insoluble residue. (C) Endo-polygalacturonase (EPG) digestion of the oxalate-soluble fraction and oxalate-insoluble residue releases RG-I. (D) RG-I is purified by size exclusion chromatography (SEC), and the resulting hypothetical spectrum is shown. (E) Schematic depicting a simplified mechanism of the activity of EPG used herein. For simplicity, the backbones and side chains of RG-I and rhamnogalacturonan II (RG-II) are represented as curved lines. EPG cleaves the α-1,4 glycosidic linkages between galacturonic acid residues within the homogalacturonan (HG) backbone. Pectin domains, including HG, RG-I, and RG-II, are believed to be covalently linked to one another via HG in the wall. Cleavage of HG by EPG releases RG-I from the pectin network [1,2].
Background
The plant cell wall is a dynamic and complex extracellular structure that surrounds all plant cells, providing mechanical support, determining cell shape, mediating intercellular communication, and regulating growth. It is primarily composed of cellulose microfibrils embedded in a matrix of hemicelluloses, pectins, proteins, and other polysaccharides [3]. Pectins are a diverse group of polysaccharides that are abundant in the middle lamella, as well as in the primary walls of dicots and nongraminaceous monocots. They play crucial roles in numerous biological processes contributing to cell–cell adhesion, cell wall porosity, hydration, and flexibility. Mutant plants with defects in pectin composition or modification often show developmental abnormalities, such as smaller rosette leaves, irregular organ formation, uneven cell expansion, and defects in pollen tube growth [1,4,5]. Rhamnogalacturonan-I (RG-I) is a major type of pectin characterized by a backbone of alternating rhamnose and galacturonic acid residues, often decorated with arabinan, galactan, and arabinogalactan side chains, that may also be modified by backbone acetylation (Figure 1).
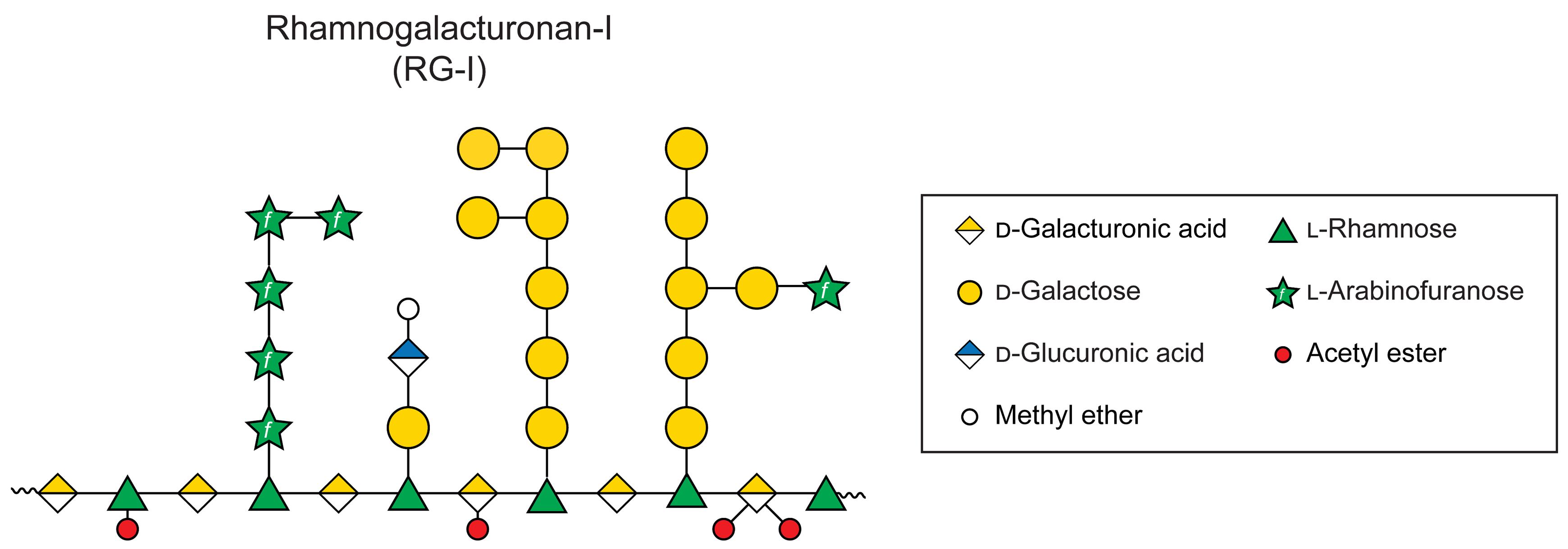
Figure 1. Representative model structure of rhamnogalacturonan-I (RG-I) sidechains. The specific structure of RG-I side chains is not known and is representative. Monosaccharide symbols used in the representative schematic structure are taken from the Symbol Nomenclature for Glycans (SNFG) from the Consortium for Functional Glycomics.
Most research on pectin has focused on homogalacturonan (HG), while the structure and function of RG-I have remained poorly understood, especially in model species. Indeed, much of our knowledge of RG-I largely comes from non-model species, which limits genetic studies of its synthesis and biological functions. RG-I is highly diverse, varying in structure by tissue, species, and developmental stage [6], and its biosynthesis likely involves a multitude of enzymes with distinct characteristics. Arabidopsis has long served as a model for studying plant cell wall structure, biosynthesis, remodeling, and function [7,8]. Thus, foundational knowledge about the structural features of RG-I in Arabidopsis is necessary for designing future experiments to gain insights into its biosynthesis and roles in plant development.
To better understand the structure and function of RG-I, it is essential to isolate this polysaccharide in sufficient quantity and purity. Isolated RG-I can be analyzed for its monosaccharide composition, glycosyl linkages, and structural features, providing insights into its diversity and biological roles. Furthermore, purified RG-I serves as a valuable substrate for enzymatic studies, enabling the characterization of pectin-synthesizing [9] and pectin-degrading [10] enzymes and the investigation of cell wall remodeling processes. This makes the development of reliable isolation protocols a critical step for advancing both fundamental and applied plant cell wall research. Here, we present a detailed protocol for isolating RG-I from Arabidopsis rosette leaves. Our protocol comprises two main steps: solubilization of pectin using ammonium oxalate and the release of RG-I through cleavage of pectin with endo-polygalacturonase (EPG) (Graphical overview). The oxalate functions as a chelating agent, sequestering divalent cations and thereby solubilizing pectin that is stabilized in the cell wall through calcium-mediated cross-links. Other chelators, such as ethylenediaminetetraacetic acid (EDTA) and 1,2-cyclohexylenedinitrilotetraacetic acid (CDTA), have also been employed for pectin extraction [11]. However, these compounds are difficult to remove by dialysis, which can interfere with downstream analyses. Following the solubilization step, pectin is digested with EPG, producing a mixture of RG-I, rhamnogalacturonan II (RG-II), and oligogalacturonides (OGAs), which can subsequently be separated by size-exclusion chromatography (SEC). Although this protocol is demonstrated using Arabidopsis rosette leaves, it has also been successfully applied to other Arabidopsis tissues as well as to various plant species [12,13].
Materials and reagents
Biological materials
1. Arabidopsis thaliana ecotype Columbia-0 (Col-0)
2. Spirizyme Excel (Novozymes, catalog number: NAPFM084)
3. Liquozyme SC DS (Novozymes, catalog number: AUP61163)
4. Endo-polygalacturonase M2 (EPG, Megazyme, catalog number: 700004232); an alternative can be found from Neogen [Megazyme endo-Polygalacturonanase (Pectobacterium carotovorum), catalog number: E-PGALPC]
Reagents
1. 190 proof ethanol (KOPTEC, catalog number: V1105HC)
2. Chloroform (VWR, catalog number: BDH83626.4)
3. Methanol (VWR, catalog number: BDH85800400)
4. Acetone (Tedia, catalog number: AS1112_001)
5. Sodium acetate (Fisher, catalog number: S220.1)
6. Ammonium oxalate (Sigma-Aldrich, catalog number: 32304)
7. Ammonium formate (Sigma-Aldrich, catalog number: 156264)
8. Sodium formate (Sigma-Aldrich, catalog number: 247596)
9. ACS-grade hydrochloric acid (HCl) 36.5%–38% (VWR, catalog number: BDH3028)
10. Miracle-Gro 20-20-20 water-soluble all-purpose food fertilizer (Jacks Classic, catalog number: 52024)
11. Metromix 830 soil (Hummert International, Berger BM7 35% Bark HP, catalog number: 10121500)
12. Vermiculite (PALMETTO VERMICULITE, A-1 Super Fine)
13. Formic acid (Fisher Scientific, catalog number: A117-50)
Solutions
1. 80% ethanol (see Recipes)
2. Chloroform:methanol (1:1) (see Recipes)
3. 100 mM sodium acetate buffer, pH 5 (see Recipes)
4. 0.5% ammonium oxalate buffer, pH 5 (see Recipes)
5. 50 mM ammonium formate buffer, pH 5 (see Recipes)
Recipes
1. 80% ethanol
| Reagent | Final concentration | Volume |
|---|---|---|
| Ethanol | n/a | 800 mL |
| ddH2O | n/a | 200 mL |
| Total | n/a | 1,000 mL |
Store at room temperature (RT).
2. Chloroform:methanol (1:1)
| Reagent | Final concentration | Volume |
|---|---|---|
| Chloroform | n/a | 500 mL |
| Methanol | n/a | 500 mL |
| Total | n/a | 1,000 mL |
Store at RT.
3. 100 mM sodium acetate buffer, pH 5
| Reagent | Final concentration | Quantity |
|---|---|---|
| Sodium acetate | 100 mM | 13.6 g |
| ddH2O | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust the pH to approximately 5 with 1 M HCl. Store at RT.
4. 0.5% (w/v) ammonium oxalate buffer, pH 5
| Reagent | Final concentration | Quantity |
|---|---|---|
| Ammonium oxalate | 35.18 mM | 5 g |
| ddH2O | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust the pH to approximately 5 with 1 M HCl. Store at RT.
5. 50 mM ammonium formate buffer, pH 5
| Reagent | Final concentration | Quantity |
|---|---|---|
| Ammonium formate | 50 mM | 3.153 g |
| ddH2O | n/a | 1,000 mL |
| Total | n/a | 1,000 mL |
Adjust the pH to approximately 5 with formic acid. Store at RT.
Laboratory supplies
1. 1.5 mL Eppendorf tubes (AXYGEN, catalog number: MCT-150-C)
2. 250 mL Erlenmeyer flask with sidearm tabulation (Corning, catalog number: 10-180D)
3. 2 mL Eppendorf tubes (Fisher Scientific, FisherbrandTM, catalog number: 13-698-792)
4. 0.2 mL PCR tubes (ASI alkali scientific ProCycle, catalog number: 25141)
5. 0.45 μm nylon filter (Thermo Scientific, catalog number: F2517-4)
6. Rotary salad spinner (Mainstays, catalog number: MS563617582S1)
7. GF/B glass microfiber filter (GE Healthcare Life Sciences, catalog number: 1820-070)
8. Bel-ArtTM plain spinbar magnetic stirring bar (Fisher Scientific, catalog number: 03-411-782)
9. SnakeSkinTM dialysis clips (Thermo ScientificTM, catalog number: PI68011PM)
10. Nylon filter mesh fabric 0.2 mm thick (Amazon OTGMGCB, catalog number: 765015439381)
11. Büchner funnel with 90 mm pore plate (GSC International, catalog number: BFP90)
12. 250 mL, 500 mL, 1 L, and 5 L beakers (Fisher Scientific, FisherbrandTM, catalog number: FB100250)
Equipment
1. Benchtop centrifuge (Eppendorf, model: 5810R)
2. Swing bucket rotor (Eppendorf, 5810/5810R, catalog number: S-4-104)
3. Freeze dry system (Labconco Corporation, model: 4.5 FreeZone, catalog number: 7960040)
4. Water bath (VWR, model: WB05, serial no. W113C0864)
5. Superdex 75 Increase 10/300 GL column (Cytiva, catalog number: 17517401)
6. Agilent 1260 Infinity II LC system (Agilent)
7. SM208 Screen Brightness Meter Screen Luminance Meter (M&A Instruments)
8. POLYTRON homogenizer (Brinkmann)
Software and datasets
1. Agilent OpenLab CDs ChemStation Rev. C.01.10 [201]
Procedure
文章信息
稿件历史记录
提交日期: Nov 7, 2025
接收日期: Dec 18, 2025
在线发布日期: Jan 5, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Zhang, L., Javaid, T. and Urbanowicz, B. R. (2026). Detailed Method for the Purification of Rhamnogalacturonan-I (RG-I) in Arabidopsis thaliana. Bio-protocol 16(3): e5579. DOI: 10.21769/BioProtoc.5579.
分类
植物科学 > 植物生物化学 > 糖类
植物科学 > 植物细胞生物学 > 细胞壁
生物化学 > 糖类 > 多糖
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












