- EN - English
- CN - 中文
Monitoring of Sperm-Independent Calcium Oscillations in Immature Oocytes of Mice
小鼠未成熟卵母细胞中非精子依赖性钙离子振荡的监测
发布: 2026年02月05日第16卷第3期 DOI: 10.21769/BioProtoc.5576 浏览次数: 165
评审: Anonymous reviewer(s)
Abstract
Repetitive increases of intracellular calcium ions (Ca2+ oscillations) control cellular functions in various biological events, including meiotic resumption after fertilization. Sperm-derived substances enter the cytoplasm of mature oocytes by sperm fusion, causing Ca2+ oscillations. Sperm-independent Ca2+ oscillations are also induced in immature oocytes isolated from the ovaries of neonatal to adult mice. The presence of Ca2+ oscillations may contribute to subsequent oocyte quality; however, its physiological role and molecular mechanism are unclear. Here, we describe a method of collecting immature oocytes from the ovaries of juvenile (12, 15, and 21 days after birth) and adult mice and monitoring their Ca2+ oscillations. Since mouse oocytes are larger than other types of cells, they are a useful model for studying spatiotemporal patterns and the mechanism of Ca2+ oscillations in various types of cells. This method can be applied to other rodents due to similarities in oocyte size and developmental processes. Furthermore, the use of various fluorescent probes enables visualization of organelle rearrangement. The mechanism of interaction between oocytes and somatic cells differs between juvenile and adult mice. Therefore, two distinct methods are employed for oocyte collection.
Key features
• Isolation of immature oocytes from juvenile ovaries [use of ethylenediaminetetraacetic acid (EDTA)].
• Isolation of immature oocytes from adult ovaries (no treatment with protease and EDTA).
• Monitoring of Ca2+ oscillations in immature oocytes.
Keywords: Ca2+ oscillations (钙离子振荡)Graphical overview
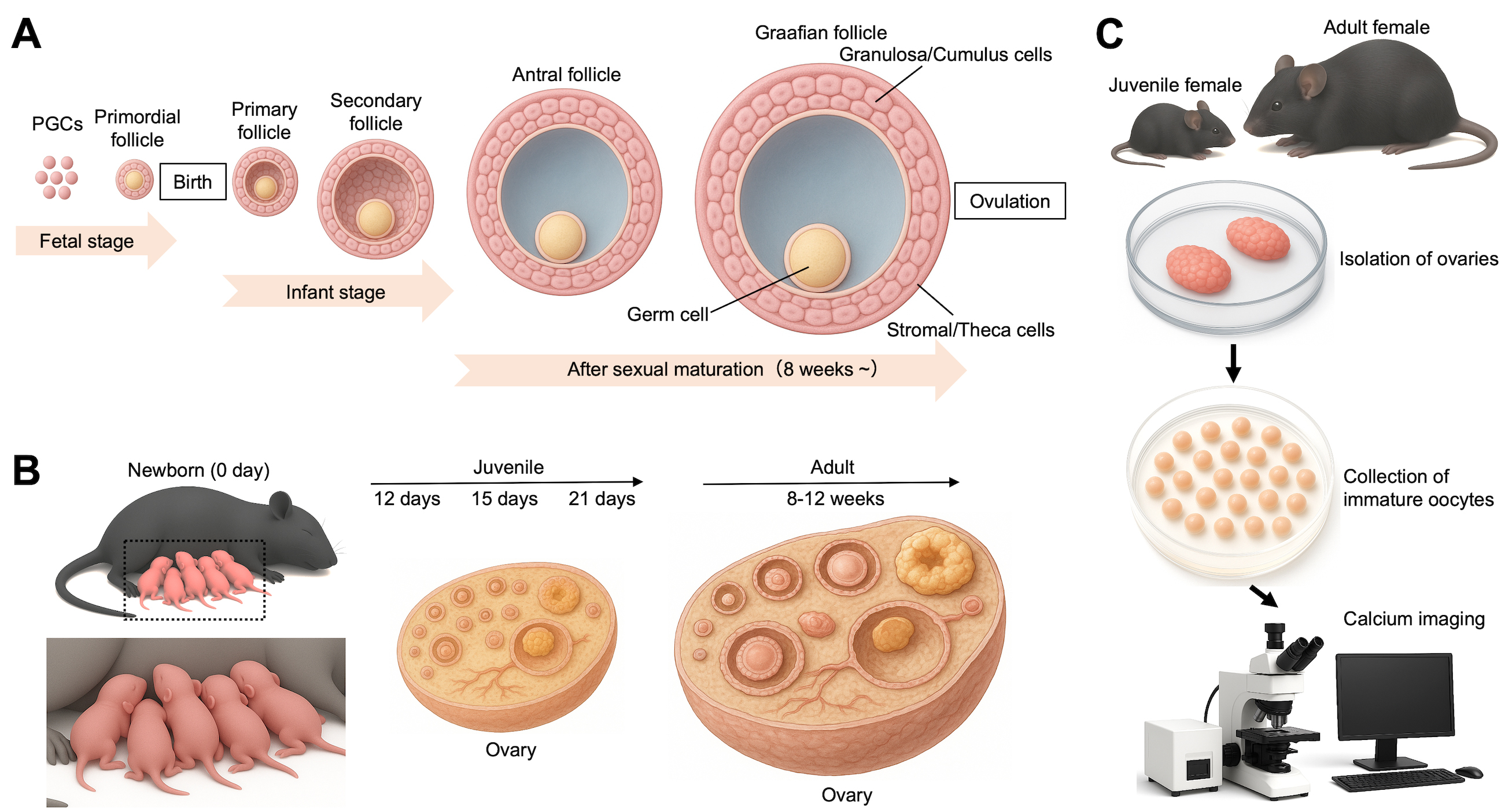
Monitoring of Ca2+ oscillations in immature oocytes from juvenile and adult mice. (A) An overview of follicular development from fetal to adult stages. Primordial germ cells (PGCs) are precursors of germline cells, including oocytes within ovarian follicles. In mammalian females, ovarian follicles form during the fetal or early neonatal period, and the pool of oocytes is considered finite at birth. (B) Representation of mice used in this protocol. After newborn mice (0 day) are raised, 12-, 15-, and 21-day-old and 8–12-week-old female mice are used here. (C) Monitoring of Ca2+ oscillations. Immature oocytes are collected from the ovaries of juvenile and adult female mice.
Background
Oscillations in the concentration of intracellular calcium ions (Ca2+ oscillations) occur in various types of cells. Ca2+ oscillations control an array of cellular processes, including cell metabolism, exocytosis, and cell cycle progression [1]. Ca2+ oscillations arise from integrated actions of multiple organelles. Mainly, the endoplasmic reticulum (ER) and mitochondria work as drivers and modulators [2]. The plasma membrane maintains the supply of Ca2+ and resets Ca2+ oscillations [3]. The nucleus interprets oscillatory signals to control long-term cellular responses [4]. These organelles cooperatively regulate Ca2+ oscillations in various types of cells, such as neural cells [5], muscular cells [6], immune cells [7], and oocytes [1]. Immediately after sperm fusion during fertilization, sperm-derived substances (sperm factors) enter the cytoplasm of mature oocytes and evoke Ca2+ oscillations, subsequently leading to meiotic resumption. Sperm-derived phospholipase Cζ1 (PLCZ1) is an enzyme that induces Ca2+ oscillations in mammalian mature oocytes. Moreover, extramitochondrial citrate synthase triggers an initial spike of Ca2+ oscillations in the presence of PLCZ1, implying that these two sperm factors work independently at least in mice. On the other hand, Ca2+ oscillations are spontaneously induced in various stages of immature oocytes [1]. Both fertilization and oocyte maturation are prerequisites for embryonic development. However, the role of Ca2+ oscillations in immature oocytes is still unclear, although preventing intracellular Ca2+ changes inhibits meiotic maturation at specific stages of oocytes [8]. Studies on how Ca2+ oscillations regulate oocyte maturation may provide new insights into the causes of female infertility. Moreover, mouse oocytes are a useful model to study the spatiotemporal mechanism of Ca2+ oscillations. This method is likely applicable to other rodents with structurally similar ovaries. Observation of calcium oscillations in oocytes is most often performed on oocytes after fusion with sperm. This method is useful for efficiently collecting oocytes within the ovary.
Materials and reagents
Biological materials
1. C57BL/6J female mice (Japan SLC, Inc.): 12, 15, and 21 days and 8–12 weeks after birth
Reagents
1. M2 medium (Sigma-Aldrich, catalog number: MR-015), store at 4 °C
2. L-15 medium (WAKO, catalog number: 128-06075), store at 4 °C
3. Liquid paraffin (Nacalai Tesque, catalog number: 26137-85, specially prepared reagent), store at room temperature (RT) and in the dark, away from sunlight
4. 70% ethanol (Yoshida Pharmaceutical Company, Ecosyoueta disinfectant solution, catalog number: 14987288980046)
5. UltraPureTM 0.5 M ethylenediaminetetraacetic acid (EDTA), pH 8.0 (Invitrogen, catalog number: 15575020), store at RT
6. Sodium chloride (NaCl) (Nacalai Tesque, catalog number: 31320-05), store at RT
7. Potassium chloride (KCl) (Nacalai Tesque, catalog number: 28514-75), store at RT
8. Calcium chloride (CaCl2) (Nacalai Tesque, catalog number: 06729-55), store at RT
9. Potassium dihydrogen phosphate (KH2PO4) (Nacalai Tesque, catalog number: 28721-55), store at RT
10. Magnesium sulfate heptahydrate (MgSO4·7H2O) (Nacalai Tesque, catalog number: 21003-75), store at RT
11. Sodium hydrogen carbonate (NaHCO3) (Nacalai Tesque, catalog number: 31213-15), store at RT
12. D-(+)-Glucose (Nacalai Tesque, catalog number: 168-06), store at RT
13. Pyruvic acid sodium salt (Nacalai Tesque, catalog number: 29806-12), store at 4 °C
14. Penicillin G potassium salt (Sigma-Aldrich, catalog number: 113-98-4), store at 4 °C
15. Streptomycin sulfate salt (Sigma-Aldrich, catalog number: S6501-25G), store at 4 °C
16: Albumin, from bovine serum, Cohn fraction V, pH 7.0 (albumin fraction V) (WAKO, catalog number: 017-23294), store at 4 °C
17. Oregon green 488 BAPTA-1 AM (Invitrogen, catalog number: O6807), store at 4 °C
18. UltraPureTM 0.5 M EDTA, pH 8.0 (ThermoFisher, catalog number: 15575020), store at RT
19. Dulbecco's phosphate buffered saline (Nacalai Tesque: 14249-24), store at RT
Solutions
1. Toyoda-Yokoyama-Hoshi (TYH) medium (see Recipes)
2. EDTA-containing PBS(-) (see Recipes)
Recipes
1. TYH medium
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaCl | - | 697.6 mg |
| KCl | - | 35.6 mg |
| CaCl2 | - | 19.0 mg |
| KH2PO4 | - | 16.2 mg |
| MgSO4·7H2O | - | 29.3 mg |
| NaHCO3 | - | 210.6 mg |
| D-(+)-Glucose | - | 100.0 mg |
| Pyruvic acid sodium salt | - | 5.5 mg |
| Penicillin G potassium salt | - | 7.5 mg |
| Streptomycin sulfate salt | - | 5.0 mg |
| Albumin fraction V | - | 400.0 mg |
| Distilled H2O | - | Up to 100 mL |
2. EDTA-containing PBS(-)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| UltraPureTM 0.5 M EDTA, pH 8.0 | 0.1 mM | - |
| Dulbecco's phosphate buffered saline | - | Up to 1 mL |
Laboratory supplies
1. Glass pipettes (Drummond Scientific Company, MICROCAPS®, catalog number: 1-000-0500)
2. 1.5 mL microcentrifuge tubes (WATSON, catalog number: 131-815C)
3. 50 mL tubes (Greiner, catalog number: 227261)
4. 10 mL plastic pipettes (FALCON, catalog number: 357551)
5. 1,000 μL pipette tips (WATSON, catalog number: 110-7-6C)
6. 200 μL pipette tips (WATSON, catalog number: 110-705C)
7. 10 μL pipette tips (WATSON, catalog number: 110-207C)
8. Kimwipes (NIPPON PAPER CRECIA, catalog number: 62020)
9. Aluminum foil (Mitsubishi aluminum, catalog number: B0093XFMQC)
10. Paper towels (ASKUL, catalog number: 1944368)
11. 35 mm dishes (IWAKI, catalog number: 1000-035)
12. 60 mm dishes (CORNING, catalog number: 351007)
13. Syringes with needles (Terumo Corporation, 1 mL syringe with 26-gauge 1/2-inch needle, catalog number: SS-01T2613S)
14. Disposable gloves (AXEL, catalog number: 61-7347-30)
15. Protective equipment (e.g., masks, goggles, and lab coats)
Equipment
1. CO2 incubator (WAKENYAKU, model: 9300E)
2. Stereomicroscope (Nikon, model: SMZ645)
3. Hot plate (NISSIN, model: NHP-M20)
4. Electronic balance (Mettler Toledo, model: Newclassic MS)
5. Gas torch (PRINCE, model: GB-2001)
6. Pipette controller (Drummond, model: Pipet-Aid XP)
7. P-1000 pipette (Gilson, model: F120602)
8. P-200 pipette (Gilson, model: F123601)
9. Micropipette (Eppendorf, model: 4920000024)
10. Mouth pipette (Drummond, model: 2-040-000)
11. Ampoule glass cutter (AXEL, model: 5-124-22)
12. Large straight scissors (Natsume Seisakusho, model: B-3)
13. Small straight scissors (Natsume Seisakusho, model: B-12)
14. Tweezers (AXEL, model: 2-529-12)
15. Precision tweezers (DUMONT, model: NO.5-INOX)
16.18-gauge needle (TERUMO, model: NN-1838R)
17. 21-gauge needle (TERUMO, model: NN-2138S)
18. 5-mL syringe (TERUMO, SS-05SZ)
19. 1-mL syringe (TERUMO, SS-01T)
20. Dispenser for liquid paraffin (Nichiryo Co., Ltd., model: 00-DP-2B)
21. Plastic cages (Clea Japan, model: CL-0103-2 Mouse TPX)
22. Confocal microscope system (Yokogawa Electric, model: CSU-Frontier-SESP1)
23. Stage top incubator (Tokai Hit, INUBG2-PPZI)
24. Highly sensitive CCD camera (Andor Technology, model: iXon EMCCD)
Software and datasets
1. R software (version 4.2.2) (https://www.r-project.org/)
2. Imaging software Andor iQ (Andor Technology: version 1.9.1)
Procedure
文章信息
稿件历史记录
提交日期: Nov 11, 2025
接收日期: Dec 18, 2025
在线发布日期: Jan 13, 2026
出版日期: Feb 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Horiike, S., Kang, W., Sato, B., Miyado, K. and Ogawa, H. (2026). Monitoring of Sperm-Independent Calcium Oscillations in Immature Oocytes of Mice. Bio-protocol 16(3): e5576. DOI: 10.21769/BioProtoc.5576.
分类
发育生物学 > 细胞生长和命运决定 > 卵母细胞
发育生物学 > 细胞信号传导 > 细胞内钙
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












