- EN - English
- CN - 中文
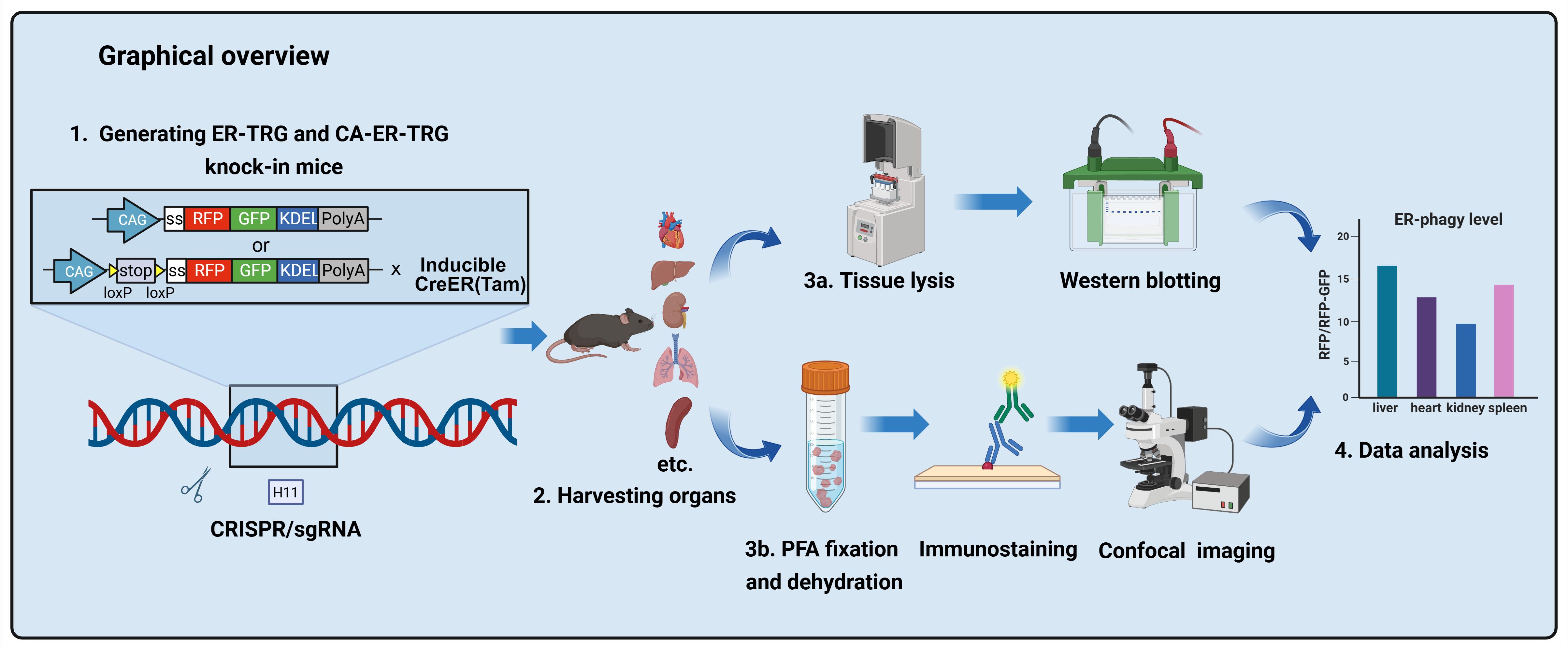
Generating ER-TRG and CA-ER-TRG Knock-in Mice and Quantitative in vivo Imaging of ER-phagy
ER-TRG和CA-ER-TRG基因敲入小鼠的构建及内质网自噬的定量活体成像
(*contributed equally to this work) 发布: 2026年01月05日第16卷第1期 DOI: 10.21769/BioProtoc.5559 浏览次数: 188
评审: David PaulKeisuke TabataAnonymous reviewer(s)
Abstract
ER-phagy, a selective autophagy process crucial for maintaining cellular homeostasis by targeting the endoplasmic reticulum (ER), has been challenging to study in vivo due to the lack of suitable spatiotemporal quantification tools. Existing methods like electron microscopy, biochemical assays, and in vitro reporters lack resolution, scalability, or physiological relevance. Here, we present a detailed protocol for generating two transgenic mouse models: ER-TRG (constitutively expressing an ER lumen-targeting tandem RFP-GFP tag) and CA-ER-TRG (Cre-recombinase-activated ER-TRG). Additionally, we outline procedures for quantitative imaging of ER-phagy in vivo, covering tissue preparation, confocal microscopy, and signal analysis. This protocol offers a robust and reproducible tool for investigating ER-phagy dynamics across various tissues, developmental stages, and pathophysiological conditions, facilitating both fundamental and translational research.
Key features
• Enables live, single-cell resolution imaging of ER-phagy dynamics across intact tissues in mice.
• Features a Cre-recombinase-activated knock-in model (CA-ER-TRG) for spatiotemporally controlled ER-phagy studies in specific cell types.
• Quantifies ER-phagy flux via pH-sensitive RFP-GFP signal ratiometry and lysosomal co-localization in vivo.
Keywords: ER-phagy (内质网自噬)Graphical overview
Background
The endoplasmic reticulum (ER) plays a pivotal role in multiple cellular functions, including protein synthesis, lipid metabolism, and calcium homeostasis [1]. To maintain its proper function, excess or damaged ER is degraded via ER-phagy, a conserved selective autophagy pathway [2,3]. Dysregulation of ER-phagy has been implicated in numerous diseases, such as cancer, neurodegeneration, and metabolic disorders, highlighting the importance of studying its in vivo dynamics [4].
Current methods for assessing ER-phagy, such as electron microscopy, biochemical assays, or in vitro fluorescent reporters, present significant limitations. Electron microscopy is labor-intensive and not well-suited for large-scale tissue analysis. Biochemical assays lack cellular-level detail, and in vitro reporters cannot recapitulate the complexity of in vivo tissues.
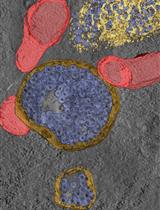
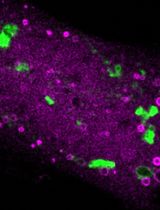
To address these limitations, we engineered ER-TRG and CA-ER-TRG knock-in mice. The ER-TRG model constitutively expresses a KDEL-tagged tandem RFP-GFP reporter that localizes to the ER lumen. This established reporter strategy leverages the distinct pH-dependent fluorescence properties of RFP and GFP to quantify autophagic flux. As the reporter is ubiquitously distributed throughout the ER network, it serves as an effective tool for measuring bulk ER-phagy [5].
While overexpression of ER-lumen tandem fluorescent proteins functions reliably as ER-phagy reporters in vitro, their in vivo efficacy for faithful visualization of ER-phagy and architecture without adverse effects remains uncertain. To address this, we construct the CA-ER-TRG model, a Cre-inducible variant, by integrating the Cre-loxP recombination system with the ER-TRG gene cassette. This approach allows for the insertion of the ER-TRG gene into the genome at a specific locus under the control of a Cre-responsive promoter. Upon introduction of Cre recombinase, the loxP sites are recombined, enabling the expression of ER-TRG in a spatiotemporally controlled manner. This precise genetic engineering strategy minimizes potential artifacts associated with constitutive reporter expression [6].
These tools enable the study of ER-phagy across different tissues, developmental stages, and stress conditions (starvation, injury), facilitating insights into its physiological and pathological roles.
Materials and reagents
Biological materials
1. H11-CAG-PMD18T (Synthesized by Gempharmatech)
2. pCW57-CMV-ssRFP-GFP-KDEL (Addgene, catalog number: 128257 [5])
3. pER-TRG (donor vector, available on request [7])
4. pCA-ER-TRG (donor vector, available on request [7])
5. AAV-PHP.eB-hSyn-Cre (commercially custom-produced, wzbio, Shandong [7])
6. C57BL/6J mice (Gempharmatech)
7. ICR mice (Charles River)
8. ER-TRG mice [7]
9. CA-ER-TRG mice (Jackson Laboratory, catalog number: 040208 [7])
10. Aldh1l1-CreERT2 mice (Gift from Prof. Tianming Gao, Sothern Medical University [8])
11. Primary hepatocytes (isolated from ER-TRG mice)
12. Liver and brain from wild-type (WT) and transgenic mice
Reagents
1. Cas9 (ACROBiosystems, catalog number: GMP-CA9S18)
2. PMSG (NSHF)
3. hCG (NSHF)
4. Hyaluronidase (AibeiBio, catalog number: M2215)
5. Mineral oil (AibeiBio, catalog number: M2470)
6. M2 culture medium (Sigma, catalog number: MR-015-D)
7. KSOM culture medium (Sigma, catalog number: MR-121-D)
8. Mouse monoclonal anti-GAPDH (Proteintech, catalog number: 60004-1-Ig)
9. Mouse monoclonal anti-RFP (Thermo Fisher Scientific, catalog number: MA5-15257)
10. Rabbit monoclonal anti-LAMP1 (Cell Signaling, catalog number: 99437S)
11. Goat anti-mouse IgG (H+L), Alexa Fluor 647 (Thermo Fisher Scientific, catalog number: A-31573)
12. HRP conjugated goat anti-rabbit IgG goat polyclonal antibody (HuaBio, catalog number: HA1001)
13. HRP conjugated goat anti-mouse IgG goat polyclonal antibody (HuaBio, catalog number: HA1006)
14. DMSO (Sigma, catalog number: D2650)
15. Agarose (Sigma, catalog number: A2576)
16. HEPES (Sigma, catalog number: 54457)
17. Potassium acetate (Sigma, catalog number: P1190)
18. DAPI (Beyotime, catalog number: C1002)
19. SAKURA Tissue-Tek® OCT compound (Sakura, catalog number: 4583)
20. Paraformaldehyde (PFA) (Aladdin, catalog number: 30525-89-4)
21. Sucrose (Aladdin, catalog number: S818049)
22. Fluoromount-G (SouthernBiotech, catalog number: 0100-01)
23. DMEM medium (high glucose) (BasalMedia, catalog number: L110KJ)
24. Fetal bovine serum (FBS) (ExCell Bio, catalog number: FSS500)
25. Penicillin-streptomycin (100×) (BasalMedia, catalog number: S110JV)
26. Collagenase Type II (Thermo Fisher Scientific, catalog number: 17101-015)
27. Collagenase Type IV (Thermo Fisher Scientific, catalog number: 17104-019)
28. Protease inhibitor cocktail (Roche, catalog number: 04693132001)
29. Phosphatase inhibitor (Selleck, catalog number: B15001)
30. Tamoxifen (MCE, catalog number: HY-13757A)
31. Corn oil (MCE, CAS number: 8001-30-7)
32. BSA (FUDE Biology, catalog number: FD0030)
33. Polyvinylidene fluoride (PVDF) membrane (Vazyme, catalog number: E801-01)
34. BCA Protein Assay kit (Beyotime, catalog number: P0012)
35. RIPA lysis buffer (Beyotime, catalog number: P0013E)
36. Tris-HCl (1 M, pH 8.0) (Sangon Biotech, catalog number: B548127-0500)
37. NaOH (Sangon Biotech, catalog number: A100583-0500)
Solutions
1. Perfusion buffer I (see Recipes)
2. Perfusion buffer II (see Recipes)
3. 4% PFA (pH 7.0) (see Recipes)
4. Permeabilization buffer (see Recipes)
5. Blocking buffer (see Recipes)
6. Tamoxifen (see Recipes)
Recipes
1. Perfusion buffer I
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| NaHCO3 | 0.075% | 300 μL |
| EDTA | 0.5 mM | 30 μL |
| HANKS (no Ca/Mg) | 100% | 30 mL |
| Total | n/a | 30 mL |
Dissolve 3.75 g of NaHCO3 in distilled water, fill up to 50 mL, and filter-sterilize with a 0.22 μm filter. Dissolve 9.306 g of EDTA·2Na·2H2O in distilled water, fill up to 50 mL, adjust pH to 8.0 with NaOH, and filter-sterilize with a 0.22 μm filter. For 30 mL of perfusion buffer I, mix 30 mL of HANKS (no Ca/Mg), 300 μL of 7.5% NaHCO3, and 30 μL of 0.5 M EDTA (pH = 8.0), and filter-sterilize with a 0.22 μm filter. Store at 4 °C and prewarm to 37 °C before use.
2. Perfusion buffer II
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| CaCl2 | 5 mM | 20 μL |
| NaHCO3 | 0.075% | 100 μL |
| Collagenase Type II | 0.5–1 mg/mL | 5–10 mg |
| Collagenase Type IV | 0.5–1 mg/mL | 5–10 mg |
| HANKS (Ca/Mg) | 100% | 10 mL |
| Total | n/a | 10 mL |
Dissolve 13.8725 g of CaCl2 in distilled water, fill up to 50 mL, and filter-sterilize with a 0.22 μm filter. Prepare fresh immediately before use. Dissolve 3.75 g of NaHCO3 in distilled water, fill up to 50 mL, and filter-sterilize with a 0.22 μm filter. For 10 mL of perfusion buffer II, mix 10 mL of HANKS (Ca/Mg), 100 μL of 7.5% NaHCO3, 20 μL of 2.5 M CaCl2, and 0.5–1 mg/mL Collagenase Type II and IV. Prepare the solution, which should appear light brown, and filter-sterilize with a 0.22 μm filter. Store at 4 °C and prewarm to 37 °C before use.
3. 4% PFA (pH 7.0)
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| PFA | 4% | 40 g |
| PBS | 100% | 1 L |
| Total | n/a | 1 L |
Dissolve 40 g of paraformaldehyde in sterile PBS and fill up to 1 L. Adjust pH to 7.0 with NaOH or HCl. Store at 4 °C.
4. Permeabilization buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Triton X-100 | 0.3% | 30 μL |
| BSA | 3% | 300 mg |
| PBS | 100% | 10 mL |
| Total | n/a | 10 mL |
Dissolve 30 μL of Triton X-100 and 300 mg of BSA in sterile PBS and fill up to 10 mL. Prepare fresh immediately before use.
5. Blocking buffer
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Goat serum | 5% | 500 μL |
| PBS | 100% | 10 mL |
| Total | n/a | 10 mL |
Dissolve 500 μL of goat serum in sterile PBS and fill up to 10 mL. Store at 4 °C.
6. Tamoxifen
| Reagent | Final concentration | Quantity or volume |
|---|---|---|
| Tamoxifen | 2% | 200 mg |
| Corn oil | 100% | 10 mL |
| Total | n/a | 10 mL |
Dissolve 200 mg of tamoxifen in corn oil by ultrasonication and fill up to 10 mL. Store at -20 °C under light-protected conditions.
Laboratory supplies
1. 100 mm cell culture dish (Vazyme, catalog number: CCD10150)
2. Cell culture plate, 12 well (Vazyme, catalog number: CCP01012)
3. Centrifuge tubes, 15 mL (Vazyme, catalog number: TCF0015A-02B)
4. Centrifuge tubes, 50 mL (Vazyme, catalog number: TCF0050A-02B)
5. Disposable serological pipette, 10 mL (Vazyme, catalog number: CPP00010)
6. 10 μL pipette tips (Kedichen, catalog number: KDC-T-10)
7. 200 μL pipette tips (Kedichen, catalog number: KDC-T-200)
8. 1,250 μL pipette tips (Kedichen, catalog number: KDC-T-1250-B)
9. 0.22 μm filter (Jetbiofil, catalog number: FPE204030)
10. 70 μm cell strainer (Jetbiofil, catalog number: CSS013070)
11. Adhesive slides (SHITAI, catalog number: 158105W)
12. Millipore centrifugal columns (Millipore, UFC501024)
13. Capillary glass tubes GC-1 (Narishige, catalog number: GC-1)
14. Capillary glass tubes G-100 (Narishige, catalog number: G-100)
Equipment
1. Transjector (Eppendorf, model: 5246)
2. Micromanipulator (Narishige, model: NT-88NE)
3. Inverted microscope (Nikon, model: Diaphot 300)
4. Micropipette puller (Narishige, model: PN-30)
5. Stereomicroscope (Nikon, model: MZ800N)
6. Micropipette grinder (Narishige, model: EG-400)
7. Microforge (Narishige, model: MF-900)
8. Confocal microscope (ZEISS, model: LSM 800 with Airyscan)
9. 37 °C Incubator (Heal Force, model: HF90)
10. Cryostat (Thermo Fisher Scientific, model: NX50)
11. Imaging scanning system (Tanon, model: 5200)
Software and datasets
1. LSM 800 Browser (for controlling the laser confocal microscope LSM 800 for image acquisition and processing, ZEISS)
2. Imaris 9.3.1 (3D and 4D biomedical image analysis software, Bitplane)
3. SPSS 17 Measurement data analysis (advanced statistical analysis software, IBM company)
4. GraphPad Prism 8.0.2 (biostatistical analysis software, GraphPad Software Inc.)
5. ImageJ (free and open-source image processing software, available at https://imagej.net)
Procedure
文章信息
稿件历史记录
提交日期: Sep 3, 2025
接收日期: Nov 20, 2025
在线发布日期: Dec 14, 2025
出版日期: Jan 5, 2026
版权信息
© 2026 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Zhang, M., Zhang, Y., Sang, Y. and Sun, Q. (2026). Generating ER-TRG and CA-ER-TRG Knock-in Mice and Quantitative in vivo Imaging of ER-phagy. Bio-protocol 16(1): e5559. DOI: 10.21769/BioProtoc.5559.
- Sang, Y., Li, B., Su, T., Zhan, H., Xiong, Y., Huang, Z., Wang, C., Cong, X., Du, M., Wu, Y., et al. (2024). Visualizing ER-phagy and ER architecture in vivo. J Cell Biol. 223(12): e202408061. https://doi.org/10.1083/jcb.202408061
分类
细胞生物学 > 细胞结构 > 细胞器
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link