Advanced Search
Protocol for the Relative Water Content Determination, Proline Content Analysis and Field Capacity Measurement to Observe Drought Stress in Capsicum chinense Jacq (Umorok) Treated with Three Levels of Biochar
Last updated date: Dec 6, 2025 Views: 418 Forks: 0
Abstract:
This study investigates the effects of three biochar levels (120 g, 300 g, and 420 g per plant) on proline content and relative water content (RWC) in Capsicum chinense Jacq. under field capacity of 45%. Leaf discs (2 × 2 cm; 0.50 g fresh weight) from mature, fully expanded leaves were collected in zipper-locked bags, transported on ice, and weighed to obtain fresh weight. Samples were then hydrated in distilled water for 4 h to determine turgid weight, followed by drying in a hot air oven at 80°C until constant dry weight was achieved, following the method of Weatherley (1950). Relative water content was calculated from fresh, turgid, and dry masses to assess plant water status and the influence of biochar application on leaf hydration under drought stress conditions. Increasing biochar levels improved RWC, while proline content decreased at higher dosages, suggesting reduced stress. The findings highlight the role of biochar in modulating physiological stress responses and improving plant resilience.
Keywords: Biochar, Capsicum chinense Jacq., Relative Water Content, Proline, Field Capacity, Stress Physiology, Drought Study in Manipur
Graphical Illustration: Capsicum chinense Jacq. Plants were subjected to drought stress at 45% field capacity, with simultaneously application of locally available biochar, except for the control; plants, which received normal watering conditions.
1.1 Experimental Setup:
The experiment was conducted using Capsicum chinense Jacq. plants grown under drought conditions having field capacity of 45%. The earthen planting pots of length 26cm, and diameter of 24cm having 6kg soil weight were used. The garden soil was mixed with compost in the ratio of 4:1. Then locally available biochar was mixed with the soil in the ratio of 2%, 5%, 7% to soil weight in those pots (Chrysargyris et al., 2020; Pudjiastuti et al., 2025). Uniform application of 0.32 g of urea, 0.933 g of triple superphosphate (TSP), and 0.64 g of muriate of potash (MOP) were added in each pot. The seeds of Capsicum chinense were collected and sown on the nursery planting beds. When the seedlings reached height of 4 cm, they were transplanted to the pots. The experiment was design in Randomized Block Design (RBD) and the investigation was done at the experimental farm of School of Agriculture and Allied Sciences, Manipur International University. The equal mixture of 50 gm of manure, 50 gm of dry cow dung and 50 gm of vermicompost were added to each pot as a natural fertilizer. The plant samples was subjected to drought stress by applying 500 ml of water for 6 days and 4 days of non- watering (moderate drought stress)(Tan & Gören, 2024). We divided the plants into 4 different types as 2% biochar, 5% biochar, 7% biochar and no biochar (Control) treatments. As for the control we applied water every day.
1.2 Field Capacity Measurement:
Field capacity (FC) determination: Field capacity is the ability of soil particles to hold as much water as possible against gravity and experimentally it is conducted to determine the watering volume. Five pieces of 250 g pot were filled with 100 g of planting media each. All the pots were watered 100 mL until they got saturated then left for 3×24 h until the water stopped dripping (Sinamo et al., 2018). The result were then weighed as the wet weight (WW). The planting media was put into the oven at 100°C. After 24 h, the planting media was taken out from the oven, cooled in a desiccator and then were weighed as the dry weight (DW). To get the average result, the experiment were replicated 5 times. After that, the field capacity (FC) of the pots experiment was calculated using the following equation (Zulkarnaini et al., 2020):
FC=![]()
Where, FC= field capacity, WW= weight water, DW= dry weight
| Replication | Wet Weight | Dry Weight (DW) | WW-DW | Field Capacity |
R1 | 128.81 | 87.43 | 41.38 | 47.32 |
R2 | 125.14 | 88.77 | 36.37 | 40.97 |
R2 | 127.66 | 87.23 | 40.43 | 46.34 |
R4 | 127.40 | 89.23 | 38.17 | 42.77 |
R5 | 128.20 | 87.61 | 40.59 | 46.33 |
The average field capacity = (47.32+ 40.97+46.37+42.77+46.33) ÷ 5
= 44.752
= 45%
1.3 Relative Water Content (RWC):
Leaf relative water content was estimated from the turgid Weight (TM) of 0.50gm of fresh leaf (FW) sample after being kept in water for 4 hours, followed by drying in hot air oven at 800C till constant mass dry mass(DW) was achieved by the methods of Weatherley,1950.
1.3.1 Materials and Equipment Required:
- Mature 3 plant leaves for each different biochar treatment.
- Zipper –locked plastic bag for each sample.
- Milton ice box (Supper chill 3 insulated ice pail 2.06 liters) for carrying sample from field to University.
- Sharp Scissors
- Scalpel or blade
- Dissecting Forceps
- Analytical balance with 0.1 mg readability
- Petri dish
- Distilled water
- Marker
- Hot air oven
Gloves
1.3.2 Procedure:
1. Plants were grown in the pots.
2. The plastic bags were weight prior to other measurements and marked them
3. The mature fully expanded leaf samples for each biochar treatments are measured at daytime (Matin et al., 1989).
4. The Capsicum leaf for each treatment was cut in a rectangular shape of 2×2 cm size weighing 0.50 gm each as fresh weights.
5. The rectangular shape sample were put in different petri dish for each treatment by marking the petri dish by a marker.
6. Each petri dish was filled with 20 ml of distilled water to get turgid weights (TW) of each sample.
7. After 4 hours TW of each sample was weight and recorded.
8. The samples were inserted in oven at 800C for 24 hours or untilled a constant weight of each sample was maintained.
9. The dry weight (DW) of each sample were recorded
10. The relative water content was calculated as (Weatherley, 1950)
RWC=
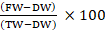
Notes:
1. Using of durable zipper-lock bags is essential to minimize the risk of moisture and liquid leakage.
2. To get reliable results working quickly and gently is essential.
3. Ensure that samples are always collected and measured in the same order to maintain procedural consistency.



Fig (1) Mature leaves collected in a plastic zipper bag, (2) The leaf discs of 0.5 gm each for turgid weight and (3) The samples prepared for oven drying
2.1 Proline Test:
2.1.1 Materials and Reagents:
1. Electronic Balance, Attogram
2. Gloves and mask
3. Beaker, Borosilicate Glass 100ml
4. Magnetic Stirrer with hot plate, Skybound
5. Test tubes, borosil 15×125,13 ml
6. Micropipette, Tarsons accupipet
7. Water bath machine, LABTRONIKS
8. Ice bath
9. Mature Plant leafs sample
10. Scalpel or blade
11. Mortar and pestle , Cole Parmer
12. Funnel, Borosil
13. Centrifuge machine, Laboratory Centrifuge Machine
14. Centrifuge tube ,15 ml
15. Cylindrical flask, BOROSIL 250:2.0ml
16. Whatman Filter paper no 1
17. L-proline,99% Purity,Crystalline,C5H9NO2,CAS 147-85-3
18. Ninhydrin, Numex Chemical Products
19. Orthophosphoric acid abt.85% LR ,Batch no G24A/1724/1907/13
20. Acetic Acid Glacial A0060,RANKEM Laboratory reagent
21. Sulphosalicylic acid 3% solution
22. Toluene, HPLC GRADE,RANKEM Toluene C6H5CH3 M.W.92.14,Product Code:T0093
23. Freezer, LLOYD, A Harvells Brand
24. Distilled Water
25. Vortex Mixer, DIRECT
26. Spectrophotometer, EI
2.1.2 Standard Curve Protocols:
1. Exact 10 mg of L-proline was weight in electronic Balance, then mixed with 100 mL of distilled water
2. Stirred the solution in Magnetic Stirrer with hot plate to create a stock solution.
3. From this stock, 10 mL aliquots of 20, 40, 60, 80, and 100 ppm standard working solutions were then prepared.
4. The 6 M orthophosphoric acid was first prepared by mixing 41 mL of 85% orthophosphoric acid with 59 mL of distilled water. The Acid-ninhydrin reagent was then prepared by warming 1.25 g of ninhydrin in a solution containing 30 mL of glacial acetic acid and 20 mL of the 6 M Orthophosphoric acid and stored in 40C.
5. Then, 2 ml of each stock were extracted and placed in separate test tubes. Separately, 2 ml of glacial acetic acid and 2 ml of the acid ninhydrin reagent were added to the test tubes. For one hour, each test tube was incubated at 1000C. The experiment was immediately terminated in an ice bath. Observe the chromophore change from yellowish to reddish due to heat.
6. Lastly, 4 ml of toluene were added to each test tube after they have cooled, and the mixture was shaken or vortexed for a minute that helped in development of a layer of red chromophore. To create a standard proline curve, the upper thick and red chromophore was removed from the test tube by micropipette and measured at 520 nm using a spectrophotometer to construct a standard curve.
2.1.3 Plant Sample Proline Estimation Protocols:
1. Fully grown leaves from every treatment were taken randomly from the pots. The leaf sample, weighing 0.5 g fresh was cut by blade and ground uniformly in 10 ml of 3% aqueous sulfosalicylic acid separately, centrifuged and filtered the plant extract.
2. In the test tube, 2 ml of filtrate were combined with 2 ml of glacial acetic acid and acid ninhydrin, and the mixture was incubated at 1000C one hour. Finish in an ice bath. Each colour of the chromophore will change from yellowish to reddish due to heat.
3. After chilling each test tube, 4 ml of toluene were added. After that, the mixture was vortexed or swirled for one minute. Red chromophore formed a coating on the surface of toluene. To generate a proline curve, the upper, thick, red chromophore at 520 nm was measured using a UV Vis-spectrophotometer. For every treatment, readings were obtained from a minimum of three replicates.


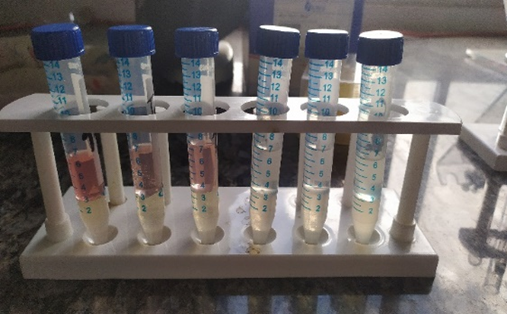
Fig (4): (a) An experimental setup for proline analysis; (b) Plant extract before water bath; (c) The picture showing red a coating on the surface of toluene
Fig (5): Standard Curve
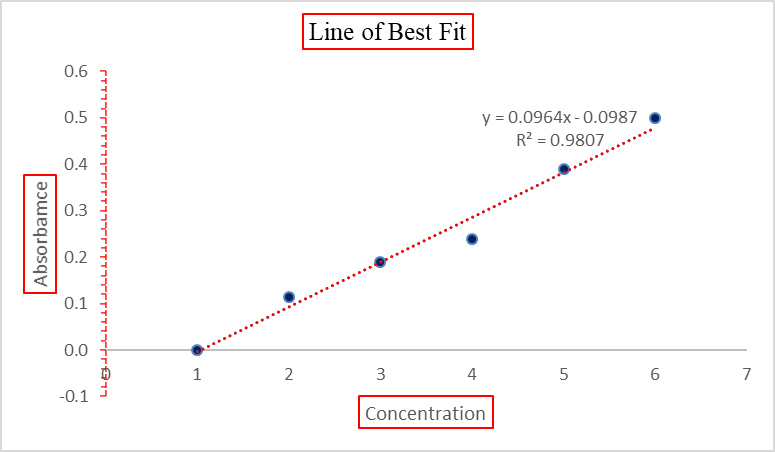
Concentration
Average
0 ppm
0.000
20 ppm
0.114
40 ppm
0.189
60 ppm
0.238
80 ppm
0.391
100 ppm
0.499
References:
1. Bates, L. S., Waldren, R. P. A., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and Soil, 39(1), 205–207.
2. Chrysargyris, A., Prasad, M., Kavanagh, A., & Tzortzakis, N. (2020). Biochar type, ratio, and nutrient levels in growing media affects seedling production and plant performance. Agronomy, 10(9), 1421.
3. Matin, M. A., Brown, J. H., & Ferguson, H. (1989). Leaf water potential, relative water content, and diffusive resistance as screening techniques for drought resistance in barley. Agronomy Journal, 81(1), 100–105.
4. Nurliana, S., Fachriza, S., Hemelda, N. M., & Yuniati, R. (2022). Chitosan application for maintaining the growth of lettuce (Lactuca sativa) under drought condition. IOP Conference Series: Earth and Environmental Science, 980(1), 12013. https://doi.org/10.1088/1755-1315/980/1/012013
5. Pudjiastuti, A. Q., Praseyorini, L., Wilujeng, R., & Cahya, U. T. W. (2025). Optimizing the application of biochar to improve irrigation efficiency and enhance the growth of chili plants in loam soil. Journal of Ecological Engineering, 26(1).
6. Tan, U., & Gören, H. K. (2024). Comprehensive evaluation of drought stress on medicinal plants: a meta-analysis. PeerJ, 12, e17801.
7. Weatherley, P. (1950). Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytologist, 81–97.
8. Zulkarnaini, Z. M., Sakimin, S. Z., Mohamed, M. T. M., Jaafar, H. B., Amnah, S., & Mellisa. (2020). Anti-transpirant application improves the drought toleranc e of fig ( Ficus carica L.) under optimization of brassinolide. Asian J Cro p Sci, 12(1), 1–11.
- Salam, S, Singh, P K, S., S J and Singh, C S(2025). Protocol for the Relative Water Content Determination, Proline Content Analysis and Field Capacity Measurement to Observe Drought Stress in Capsicum chinense Jacq (Umorok) Treated with Three Levels of Biochar. Bio-protocol Preprint. bio-protocol.org/prep2880.
Do you have any questions about this protocol?
Post your question to gather feedback from the community. We will also invite the authors of this article to respond.
Share
Bluesky
X
Copy link
